Related articles: Cohort statistics, Innate immunity, Research into protective effect of Olmesartan on various organs

Table of Contents
Survey of Marshall Protocol patients with autoimmune diagnoses (2008)
At the 6th International Congress on Autoimmunity in Porto, Portugal, Capt. Tom Perez MPH gave a presentation entitled Bacteria induced Vitamin D Receptor Dysfunction in Autoimmune Disease: Theoretical and practical Implications for Interpretation of Serum Vitamin D Metabolite Levels. The data in the presentation was based on a survey conducted over the course of 2007 and 2008 of 100 Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. (MP) patients with autoimmuneA condition or disease thought to arise from an overactive immune response of the body against substances and tissues normally present in the body diagnoses.
Patients were asked if the severity of their symptoms had changed while on the MP and, if so, how much. Improvement was reported by symptom.
The results of the survey indicate that the Marshall Protocol resolves symptoms of autoimmune disease over the course of multiple years.
Methods
Joyce Waterhouse, PhD created an 18-question survey in 2007. Surveys were distributed via email to 150 patients in the late part of 2007 and early part of 2008. These 150 patients were selected from the forum membership at large due to having reported at least one Autoimmune diagnosis.
Of the 115 surveys returned, 100 met the criteria for inclusion in the survey. Patient surveys were excluded for the following reasons:
- unconfirmed diagnosis (n = 9)
- less than one month on Protocol (n = 1)
- insufficient data/language problems (n = 1)
- severe noncompliance (n = 4)
Multiple efforts were made to follow up with people who had not completed the survey.
As this data was to be presented at the 2008 Congress on Autoimmunity, the survey only included persons on the Marshall Protocol who had a primary or secondary diagnosis of autoimmune disease. This exclusion criterion left out a number of patients with non-autoimmune diagnoses including those patients who only had fibromyalgia.
Responses from patients who discontinued the MP prior to the survey were also included (n = 3).
The small size of the survey does not lend itself to providing enough data about any particular condition to make strong assertions, but does offer provocative data that invites further research.
There were 100 patients with Autoimmune diagnoses who filled out surveys (not counting those who only had fibromyalgia). This reflects an approximately 75% response rate to the survey. Thus, 25% of the people who were sent surveys did not respond. We don't know if they are on the MP still or not, or how they are doing in most cases – for all I know they might not even have received the survey.
The response rate (percent who completed the survey of those who were sent it) among those with only fibromyalgia (from the list of conditions we surveyed) was lower – 50%. I speculate that the lower response rate may be that they think they have more options, as there are so many alternative medicine approaches that are marketed to them and so perhaps they feel less willing to stick out the IP. I don't think we can conclude it is necessarily that they have more trouble or something – we just don't know.
Joyce Waterhouse, PhD
Results
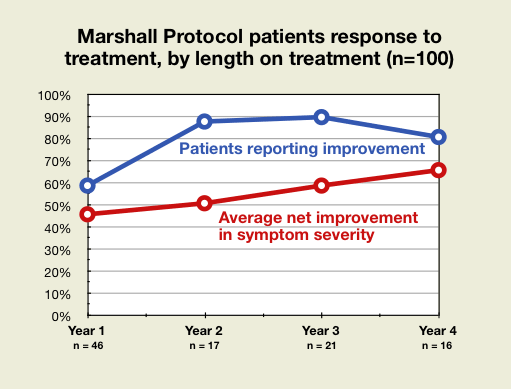
General response to treatment
MP patients reported a year-by-year increase in average net improvement in symptom severity. The number of patients reporting improvement who have been on the treatment for any given length of time also had an upward trend.
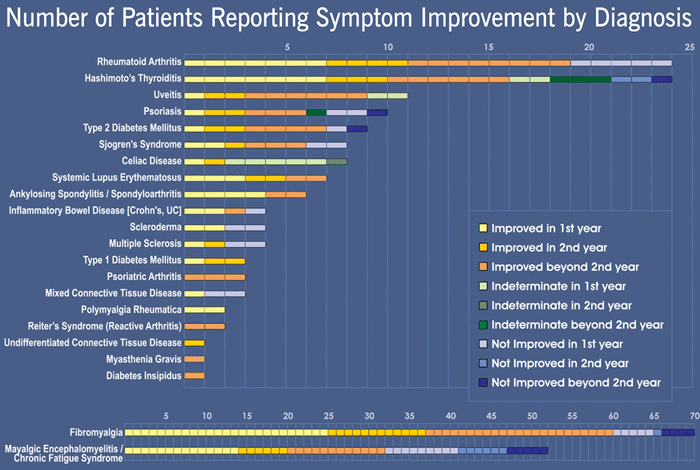
Symptom improvement by diagnosis
The following chart illustrates the proportion of patients with a given disease who reported they have improved.
Note that one cannot simply add up the numbers on the chart. Some patients had multiple diagnoses and would be counted for each. If they had thyroiditis and fibromyalgia for instance, and both conditions had improved, they would show as improved cases in both parts of the chart. If one improved and the other did not, they would show up as improved for one and not for the other.
Also note that the numbers for “not improved” with FM or CFS include those whose symptom level had not changed (a little different than the autoimmune diseases reported in the upper part of the chart).
Symptom level change on olmesartan alone
64 patients provided a response to this question on the survey. The question asked about changes in symptom level when on Benicar only (at the start of the Marshall Protocol).
- 30% reported their symptom level was the same when on Benicar alone
- 39% reported their symptom level was better when on Benicar alone
- 31% reported their symptom level was worse when on Benicar alone
Changes in inhalant allergies
22 patients who had inhalant allergies prior to starting the Marshall Protocol (and having been on the protocol 18 months or longer) provided these answers about changes in response to inhalant allergens.
- 77% reported their inhalant allergies were better (17/22)
- 14% reported their inhalant allergies were the same (3/22)
- 9% reported their inhalant allergies were worse (2/22)
Notes on interpretation
- The responses to the survey were a “snapshot in time” – various patients with various diagnosis at a specific point in time during treatment. Broad generalizations may not be possible, but the survey answers have already been meaningful to those who are considering options for treatment. Especially for rheumatoid arthritis and uveitis where the number responding was higher and the response has been very favorable.
- The limited size of the sample, 100 patients, introduces a certain amount of random variability. One could perform a statistical test on the slight drop between patients in year 3 or year 4 and find that there's no meaningful statistical difference, because the drop only represents one or two patients. A larger sample could offer more reliable data on the true rate of improvement.
- The patients on the MP are self-selected. Arguably, many of them were so sick that they were driven to start the MP as a measure of desperation. It would be reasonable to expect that a person with less severe disease symptoms would take less time on the MP.
The average duration of symptoms for this group of patients is 19 years. Many in the cohort had unsuccessfully tried a variety of other approaches, including immunosuppressants prior to initiating The Protocol.
Capt. Tom Perez, MPH