Related article: Finding a physician

This is an old revision of the document!
Table of Contents
Helping a reluctant physician to become comfortable with the MP
Although it has become less of a challenge as the work of Autoimmunity Research FoundationNon-profit foundation dedicated to exploring a pathogenesis and therapy for chronic disease. has gained acceptance, finding a physician willing to prescribe the Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. (MP) can sometimes present a challenge for patients. Those patients who have convinced their physician to employ the MP take a number of measures to make their physician comfortable with prescribing the therapy.
Those patients whose prospective doctors refuse to put them on the MP or impose excessive and unnecessary preconditions may be best advised to look elsewhere.
Practice empathy
Convincing the skeptical physician to prescribe the Marshall Protocol begins with a dose of empathy.
Doctors tend to be overworked and have a limited amount of time to devote to each patient – in the United States, typically 15 minutes for routine visits. Also, doctors pay substantial sums of money to insure themselves in the event of a lawsuit, premiums which rise if and when they are sued.
Deviating from the “standard of care,” no matter how ineffective that care may be, introduces a certain element of perceived risk – a risk some doctors are very reluctant to take. Even the question of learning about a new therapy involves a certain investment of time that many doctors are unwilling or reluctant to make.
Then there is the issue of perceived patient arrogance. Imagine spending $150,000 and four years of intensive learning and training only to be told by patients that what they discovered on the Internet trumps anything you learned in medical school.
Given all this, a pleading or heartfelt request is often not good enough.
Patients who are able to project confidence, competence, and self-sufficiency increase the odds of finding a doctor willing to use the Marshall Protocol. Emotional stability and an appeal to scientific concepts also help.
Offer materials to help address concerns
Related article: Physicians' concerns about the Marshall Protocol
- If it might help, offer evidence that the MP works and is safe. One option is to share some of the Autoimmunity Research Foundation's recent Publications and Presentations.
- Offer the Protocol Guidelines.
- Ask, “What, if any, concerns do you have about this treatment?” Listen to the answer(s).
- Offer to gather materials or take actions which attend to those concerns, many of which are addressed in the article, Physicians' concerns about the Marshall Protocol.
Offer a compromise
If a doctor remains reluctant, and working with him or her is worth the effort, offer to make a compromise. For example, some patients have said they would begin treatment with a therapeutic probeA brief trial of the Marshall Protocol to see if it will generate an immunopathological response. The "gold standard" for testing whether a patient is a good candidate for the MP., a brief trial of the MP to demonstrate efficacy. Others have begun olmesartan (Benicar)Medication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor. more slowly than they otherwise would have to address the relatively unfounded concerns about olmesartan's safety. Other patients have sold their doctor on prescribing the Marshall Protocol by signing a consent to treatment form.
Consent to treatment form
Some physicians may want to formalize in writing that the patient understands the risks of therapy. A physician may want a patient to sign a “Consent to Treatment” form, to make sure that they understand and accept any risks associated with treatment using the Marshall Protocol.
The following is a typical minimal text based on standard language for this kind of form but a given doctor may want to make changes, or ask you to use a similar document created specifically for their practice.
1. I, —–, of —-, do hereby give my consent to treatment with an innovative protocol from the Autoimmunity Research Foundation Inc. I understand that the treatment will involve the use of an Angiotensin Receptor Blocker (Benicar) at higher-than-hypotensive doses and a variety of pulsed low-dose antibiotics as outlined in the protocol guidelines. I agree to limit my own exposure to light (if I become photosensitiveAbnormal sensitivity to sunlight and bright lights. Also referred to as "sun flare" or "light flare.") and Vitamin-D, as described in the guidelines. This treatment will continue until various blood markers of inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue., also listed in the guidelines, return to normal ranges and/or until symptoms are resolved. This may take up to three years, possibly longer, depending on my individual response to the treatment. I have made my decision voluntarily and freely, and I am of sound mind.
2. I appreciate that there are certain risks associated with this treatment including the risk of severe immunopathologic reactions, and I freely assume these risks. I also understand that there are possible benefits associated with this treatment, primarily a return to normal health. However, I accept that there is no certainty that I will achieve these benefits and no guarantee has been made to me regarding the outcome of this treatment.
3. The alternatives to this treatment have been explained to me.
4. Any questions I have had regarding this treatment have been answered to my satisfaction.
5. In authorizing my physician, —-, to order this treatment, I understand that s/he may be assisted by other health professionals including Trevor Marshall, PhD, his colleagues, and such others as s/he considers necessary in my care. I agree to their participation in my care.
6. To attest to my consent to this procedure, I affix my signature to this consent:
<html>_ </html>Signature of patient
<html>_ </html>Date and time
Patients experiences
Use words wisely
Lil Sheep, MarshallProtocol.com
Be prepared
What I did was have a one-page summary of important information with URLs and a bunch of printouts that I could hand the doctor in response to anticipated questions. I only handed them over if a question was asked and was prepared to go home with any of them that did not answer a specific question asked. I also provided a list of what I needed from him to begin the protocol. Because he was a new doctor, I also had an activity matrix and a symptom matrix so that he could be apprised on the extent to which my illness debilitated me. Some people would say I had too much information and could have overwhelmed the doctor, but this doctor took the time to go through the symptom and activity matrix first, then reviewed the one-page summary, and gladly accepted each handout that I gave him in response to a question. (And I think the fact that I didn't throw everything at him at one time helped.) By the end, he was fairly confident from everything that I said and did (my organized approach) that I had a real handle on the MP and could get most of the support I needed on this site even though he expressed a real commitment to become knowledgeable.
eClaire, MarshallProtocol.com
I love it when a patient comes to me with a fully researched and logical presentation about a new course they are interested in. If it makes sense and I do not think it is dangerous, I usually go along with it. Do your homework, practice your presentation on friends, bring in papers to read (we love those, gives us the safety net of published articles) and give it your best shot. If your doc is not willing, try again next visit. If that doesn't work, find another doc.
madwolf, PA, MarshallProtocol.com
Here is what I did, and my doctor agreed to do the MP with me.
When I went to my doctor, I took notes with me, most importantly, I listed for him the lab tests needed: for me, I had decided 25-D; 1,25-D and thyroid panel (due to my thyroid problems). Then, I listed the prescriptions I would need from him: (1) Benicar, 40 mg, #120. Take as Directed. No substitutions. and (2) minocycline, 50 mg, #90. Take as directed.
In addition, I wrote up my own half-page summary of the MP. (My doctor really liked that.)
What I did was to make it as easy as I possibly could for my doctor to say yes rather than no. When you can make it easier for someone to say yes than for him/her to say no, the person usually does what you want (yes, even doctors!). So, my suggestion would be to print out the general information, include a very short summary, and address any concerns which apply specifically to you, as opposed to other people on the MP.
I mailed most of the information (a big, heavy envelope) to my doctor three weeks before I saw him, but I also brought him my very short summary, the list of lab tests (which he copied onto the order form) and the list of prescriptions and exactly how to write them (he copied what I had written).
I would add one other suggestion, and that would be to approach your doctor in a manner which suggests that you are expecting him/her to agree to do this. I told my doctor that I really wanted to do this, and that it had given me more hope than anything had in years. I do think this helped to convince him.
Patricia, MarshallProtocol.com
Well, good news! I prepared two notebooks, one with my letter, briefly giving the history of my Lyme-like malady and what I have tried, leading up to stumbling on the MP. A four point quick why MP works (turning the innate immune system back on, low dose special antibiotics that target L-form bacteriaDifficult-to-culture bacteria that lack a cell wall and are not detectable by traditional culturing processes. Sometimes referred to as cell wall deficient bacteria. folate production, managing IP, and lifestyle changes to avoid contraindicated food, supplements, drugs, and light). She was surprised that Benicar was a powerful VDR agonistA substance such as olmesartan (Benicar) or 1,25-D which activates the Vitamin D Receptor and transcribes the genes necessary for a proper innate immune response.. A page on thoughts about the MP (short, to the point). A page on is the MP applicable to me? Mentioned the mini-trial, then proposed a trial with Benicar and Minocycline, and lab test for 25 D and 1,25 D (made note of 1,25 D prep precautions).
I then showed her the phase 1 protocol, the paper on the therapeutic probe, managing IP, special extreme Herx protocol, next section covered safety of Benicar at 160 mg / day (she was really ready to know about that), the section on MP science, and physician resources. I had it set up with tabs and an index. In the larger notebook I had included the published articles and some conference transcripts. She was delighted when I gave her both notebooks, gave me prescriptions for Benicar and the Minocycline, and the order for the lab tests. I was in shock how receptive she was, but she was really bright and open-minded.
She did relate that her mother had been battling Lyme disease and she readily saw that MP was likely applicable to many other diseases.
I have started on the Benicar today with some samples she gave me. I checked with the pharmacy and Medco will allow 30 tabs of 40 mg / month with a co-pay, and I have 180 on order from an online pharmacy (slow but major savings). Lab tests are done, I just need to get the results in the next couple of days (maybe Monday). NOIRsSpecial sunglasses worn by Marshall Protocol patients to block light. should be here tomorrow, I'll wait in my bat cave, hat and sunglasses on.
So there you have it, miracles do happen thanks to the MP resources, all the hard pioneering work by TM, staff, and trail blazers, and the mysteries of the universe.
Thanks, I am on my way (and with an in network physician 3 miles away!).
mvanwink5, MarshallProtocol.com
Internalize scientific evidence
Doctors balk when they are scared, it's been my experience. [For example,] remind and challenge your doctor that there are no studies to support the notion that Prednisone changes the long-term course of sarcoidosis. Tell him your goal is long-term life and ability to enjoy it, not a stop-gap to slow your decline.
Belinda, MarshallProtocol.com
Be clear about your intentions
I think the words I finally had to use were, 'I will risk dying of sarcoidosis before I will accept the risks associated with taking Prednisone.' Then remind the doctor that you want to continue on the plan to implement the MP.
Belinda, MarshallProtocol.com
Speak confidently and without defensiveness
It's a matter of semantics. If you sound like you are apologizing for using a drug in an unconventional manner, merely playing around with some side-effect of its use, that sounds too experimental. You need to word your presentation in an authoritative way, (on a poster or anywhere doctors read it,) so that they perceive they are hearing about an alternative medical use; hence, the new Protocol using an established medication.
Personally, as a professional wordsmith, I suspect that your battle with the medical establishment is often based on emotive argument, as opposed to being based on unbiased examination of the science, if you catch my meaning. (How often do phrases like 'snake-oil' and 'witch-doctoring' enter the conversation?) It's all in how you market yourself. If this was a marketplace (which it is) and you were trying to sell your protocol (which I suppose you are) you'd be looking at who your market is and what they want.
Claudia, MarshallProtocol.com
Be firm
Skepticism aside, you control who you hire for health care. Taking responsibility for myself, I read the data and determined that my life was the one at most risk if a health care provider was skeptical (and mine was). I was ready to take my business to a neighboring state if necessary. Fortunately, it was not. With help from the Request for Doctors option on this board, I found a new doctor with a delightful spirit of adventure and curiosity, and he wasn't ready to say current consensus had already figured out my fate.
jrfoutin, MarshallProtocol.com
Never give up, never surrender! This is your life that's at stake, not your doctor's. Find someone who respects you!
Jillian, MarshallProtocol.com
Ascertain if reluctance is a provider problem or a bureaucracy problem
In Australia, physicians must now approach Canberra (federal bureaucracy) for permission to prescribe off-label.
That is what I was told, so I approached my local doctor in some trepidation armed with a bunch of references from the article on Science of OlmesartanMedication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor. Also known by the trade name Benicar. and said “I think you will need these to support a prescription for off-label Olmetec”.
He simply smiled and wrote me 2 prescriptions: One for 40mg, One for 20 mg !
I expect some other countries also have centralised control, in which case patients may need to research medical reasons other than MP, why they should take Olmesartan in preference to some more usual prescription.
Specific research
OLMesartan references to print out; for patient with doctor not a member of MPSS include information about dose relating to body weight, and dose timing
Dose timing →
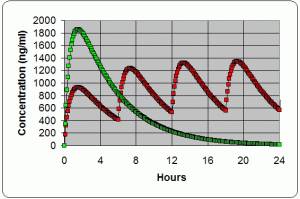
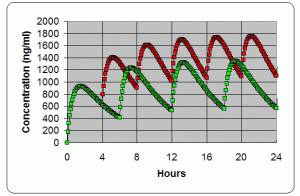
The ROADMAP study will answer the question whether an ARBA drug which is an angiotensin receptor blocker. One of the ARBs is olmesartan (Benicar). Not all ARBs activate the Vitamin D Receptor. can prevent or delay the onset of microalbuminuria and whether this translates into protection against cardiovascular events and renal disease. 1) PMID 16508590
short extract for each research study follows:
Benefits of RAS blockade with olmesartan treatment are sustained after study discontinued. 2) PMID 24772521
Olmesartan significantly reduced myocardial infarct size and improved LV contractility at a dose (3 mg/kg) with systemic vasodilating effects but not at a lower dose (0.3 mg/kg) without hemodynamic effects.(in rat)3) PMID 20074257
Inhibition of renin-angiotensin system attenuates periadventitial inflammation and reduces atherosclerotic lesion formation. 4) PMID 19304450
Olmesartan and Bay11-7082 inhibit the MCF-7 cells growth indicating RAS and NF-kappaBA protein that stimulates the release of inflammatory cytokines in response to infection pathway blockade lead to cytotoxicity & apoptosis induction against tumour cells in breast cancer 5) PMID 26138656 10)
Alzheimer's disease and dementia1 6) PMID 20068258
prevent migraines 7) PMID 12503978
inhibit liver fibrosis and aid liver healing 8) PMID 12871826
6 mg/kg olmesartan reduces the inflammatory process and bone loss in rats 9) PMID 23775504
protect the mitochondria from age-associated damage from oxidation 10) PMID 12709417
reduce liver fibrosis 11) PMID 19303015
treatment of anxiety and stress-related disorders 12) PMID 15837532
inflammation in myocarditis 13) PMID 16336207
C-reactive protein, one of the acute phase proteins that increase during systemic inflammation 14) PMID 16939632
cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. damage 15) PMID
in circadian rhythms between HR and MAP in CKD: Synchronization between the two rhythms was progressively lost as renal function deteriorated, and Olmesartan partly restored the synchronization 16) PMID 23511341
renal protective effects of olmesartan may be better than those of other ARBs 17) PMID 23511341
olmesartan may uniquely increase urinary ACE2 level, which could offer additional renoprotective effects 18) PMID 24842388
* treatment with olmesartan inhibited bone loss 19) PMID 25363367
* olmesartan protects endothelial cells against oxidative stress-mediated cellular injury 20) PMID 25904217
* decreases viability of malignant cell lines21) PMID 28666209
* carotid IMT and BP decreased similarly with olmesartan and atenolol; but only olmesartan reduced the volume of larger atherosclerotic plaques 22) PMID 19124398
* improvement of Plasma Biomarkers after switching stroke patients from other Angiotensin II Type I Receptor Blockers to Olmesartan 23) PMID 25891757
* improvement of glycemic control & insulin resistance was only observed in olmesartan group 24) PMID 23303198
* OLM substantially delayed the development of left ventricular remodeling in type 2 diabetes 25) PMID 25275251
* prevention of microalbuminuria in patients with type 2 diabetes and hypertension 26) PMID 22418908
Recent studies showed treatment with olmesartan inhibited bone loss 27), PMID 25363367
olmesartan protects endothelial cells against oxidative stress-mediated cellular injury 28), PMID 25904217
decreases viability of malignant cell lines 29), PMID 28666209
carotid IMT and BP decreased similarly with olmesartan and atenolol; but only olmesartan reduced the volume of larger atherosclerotic plaques 30), PMID 19124398
Observations suggest a positive role in a potentially lower rate of coronary atheroma progression through the administration of olmesartan, an angiotension-II receptor blocking agent, for patients with stable angina pectoris. 31) PMID 20202514
This study showed that olmesartan reduces angiotensin II and aldosterone levels more effectively than azilsartan. 32) PMID 27086671
improvement of Plasma Biomarkers after switching stroke patients from other Angiotensin II Type I Receptor Blockers to Olmesartan 33), PMID 25891757
improvement of glycemic control & insulin resistance was only observed in olmesartan group 34), PMID 23303198
OLM substantially delayed the development of left ventricular remodeling in type 2 diabetes 35) PMID 25275251
Long term treatment Data suggest 40 & 80 mg olmesartan are able to significantly remodel & destiffen the arterial wall material during long-term treatment, partly independently of blood pressure, compared with 20 mg. hyper.ahajournals.org/content/early/2014/07/07/HYPERTENSIONAHA.114.03282.reprint 36) PMID 25001274
Read more
- Second-guessing the consensus on vitamin D – a critical analysis of research used to support increasing population-wide levels of vitamin D supplementation
- Children born and living without sunlight – In 2012, it was learned that 27 children had been living underground as members of a Muslim sect. Many had lived there for their entire lives and had never seen daylight. The conditions of these children was pronounced as “satisfactory” by pediatricians.
- Vitamin D Intolerance – “I get sick as a dog.” Lyme and autoimmuneA condition or disease thought to arise from an overactive immune response of the body against substances and tissues normally present in the body patients commiserate with one another over feeling worse after taking vitamin D.
- The Truth About Vitamin D – plain language summary of the evidence for vitamin D, as of 2011
Science behind olmesartan (Benicar)
Related article: Health maintenance and Olmesartan
Patients on the Marshall Protocol (MP) take olmesartan (Benicar)Medication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor., a drug whose actions are well known, every six hours. In general, olmesartan tends to be prescribed for its antihypertensive properties due to the fact that is an angiotensin receptor blocker. A growing body of research supports the use of olmesartan as a part of a curative therapy for chronic disease.
For the purposes of the MP, olmesartan has two primary actions: it reduces inflammation by blocking the Nuclear Factor-kappaB cytokine pathway and it is an agonist of the Vitamin D ReceptorA nuclear receptor located throughout the body that plays a key role in the innate immune response. (VDR). As a VDR agonistA substance such as olmesartan (Benicar) or 1,25-D which activates the Vitamin D Receptor and transcribes the genes necessary for a proper innate immune response., olmesartan uniquely activates the innate immune responseThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease.. Research supports the safety of the doses used by MP patients.
Olmesartan has minimal interactions with other drugs and is one of the safest drugs on the market.

