Main article: Environmental causation of disease
Table of Contents
Autoimmune theory of disease
Autoimmune diseases are thought to arise from an overactive immune response of the body against substances and tissues normally present in the body. The autoimmune disease theory has yet to present a satisfactory reason, evolutionary or otherwise, why an immune system would attack human tissue.
Conversely, the Marshall PathogenesisA description for how chronic inflammatory diseases originate and develop. explains that so-called “autoantibodies” are merely antibodies generated in response to pathogenic bacterial cells that have been destroyed as a result of an active immune response – in essence, collateral damage.
At least forty different chronic diseases are suspected or accepted as being caused by an autoimmuneA condition or disease thought to arise from an overactive immune response of the body against substances and tissues normally present in the body response. According to Yehuda Shoenfeld, when it comes to autoimmune disease, “Everything is infectious until proven otherwise.”
Confusion about how autoimmunity occurs
A number of theories attempt to explain why antibodies are present in certain diseases, why the body might attack itself:
- Clonal Anergy theory
- Idiotype Network theory
- Clonal Ignorance theory
- Suppressor population theory
- Clonal Deletion theory
The last of these theories was first proposed by the 1960 Nobel laureate in medicine, Frank M. Burnett, some fifty years ago. The variety and age of these theories suggests how wide open the field is and has been.
Autoimmune patients show signs of being immunocompromised
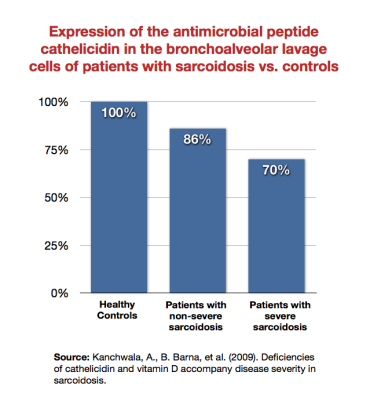
- sarcoidosis – Barna, Kanchwala, et al. showed that patients with sarcoidosis expressed the antimicrobial peptide cathelicidin Family of antimicrobial peptides found primarily in immune cells and transcribed by the Vitamin D Receptor. less than healthy subjects, and that sicker sarcoidosis patients expressed it least of all.1) 2) This was true even despite “healthy” levels of 1,25-DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol.. Wiken et al. showed that there was a reduced TLR2A receptor which is expressed on the surface of certain cells and recognizes native or foreign substances and passes on appropriate signals to the cell and/or the nervous system. mRNA expression in patients with Lofgren's syndrome (a type of acute sarcoidosis).3) Note that TLR2 (which the Marshall Pathogenesis theorizes is downregulated in autoimmune disease states) is expressed by the VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response..
- Crohn's disease – Chamaillard concluded in 2011 that “clinical studies have linked the defective expression of both α- and β-defensin to the reduced killing of certain microorganisms by the intestinal mucosa of patients suffering from ileal and colonic Crohn's disease (CD), respectively.”4) Wang et al. have demonstrated in Crohn's patients a decline in expression of key antimicrobial peptidesBody’s naturally produced broad-spectrum antibacterials which target pathogens. including cathelicidin and Beta-defensin An antimicrobial peptide found primarily in immune cells and transcribed by the Vitamin D Receptor.-2.5) Consistent with the Marshall Pathogenesis, the Wang group points to the Vitamin D ReceptorA nuclear receptor located throughout the body that plays a key role in the innate immune response. as being important in Crohn's patients.
Conversely, skin diseases like psoriasis6) and cutaneous lupus7) have higher expression of AMPs. This immune response protects the skin of the symptomatic host from further bacterial colonization.
"Autoantibodies" are produced in response to microbial DNA
The complex interactions between the innate and adaptive immune systems are largely indeterminate – they have evolved over eons, and many of the interactions make no sense when looked at individually. It is a huge mistake to think the immune system as something designed to protect the human body. It evolved over eons as a group of functions which was helpful in survival, and it was not pre-designed so that all immune functions work sensibly and deterministically.
Trevor Marshall, PhD
Autoimmune diseases are characterized largely by the presence of autoantibodies. While autoantibodies were reported over a century ago, many scientists at the time were unwilling to accept the possibility that the immune system attacks its own cells. Ehrlich argued that autoimmunity was not possible and proposed the theory of horror autotoxicus to describe the body's innate aversion to immunological self-destruction by the production of autoantibodies.
Now that humans are understood to be the product of multiple genomes, increasing evidence supports Ehrlich's view. When an innate immune system is forced to respond to a chronic microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms., the resulting cascade of chemokines and cytokinesAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. will also stimulate an adaptive response. Antibodies are notoriously polyspecific, and the likelihood that antibodies generated to target metagenomic fragments will also target human proteins (target “self”) is finite.
A litany of research implies a re-evaluation of the “autoantibody.” Recently researchers have shown that certain autoantibodies are created in response to several well-studied pathogens and in a variety of states.
- rheumatoid arthritis – Casali and Slaughter found that in humans, EBV is a polyclonal B cell activator, and in vitroA technique of performing a given procedure in a controlled environment outside of a living organism - usually a laboratory. transformation with EBV results in production of rheumatoid factor (RF).10) 11) Possnett et al. argues that high titers of RF are associated with severe rheumatoid arthritis but also appear in a number of other diseases including viral, bacterial, and parasitic infections.12) Maturation of RF can be initiated by chronic infections.13) For example, patients with subacute bacterial endocarditis, which is frequently tied to the presence of Streptococcus, also often present with high levels of RF.14) Williams et al. showed that once the offending infectious agent is removed with antibiotic therapy, the RF disappears.15)
- idiopathic thrombocytopenic purpura (ITP) – is mediated by what are considered to be anti-platelet autoantibodies. However, Asahi et al. found that eradication of H. pylori is effective in increasing platelet count in nearly half of ITP patients infected with the bacterium.16) Barzilai and team also found that Hepatitis B shares amino acid sequences with different autoantigens, further suggesting that so-called autoantibodies may actually be created in response to pathogens.17)
- Crohn's disease – Crohn's disease is classified as an autoimmune condition based largely on the presence of perinuclear anti-nuclear cytoplasmic antibodies (pANCA) in patients with the disease. Yet recently two major species of proteins immunoreactive to pANCA were detected in bacteria from anaerobic libraries, implicating colonic bacteria as a possible trigger for the disease-associated immune response.
Patients without autoimmune disease have autoantibodies during infection
Autoantibodies have been detected in patients without autoimmune disease during periods of infection. Berlin et al. collected sera from 88 patients with acute infections (41 bacterial, 23 viral, 17 parasitic, and 7 rikettsial.18) Elevated titers of autoantibodies including annexin-V, prothrombin, ASCA, ANA, or antiphospholipid antibodies were detected in approximately half of the subjects, with 34 individuals harboring elevated titers of at least two “autoantibodies.”
An increasing number of studies also show that what are currently perceived as autoantibodies can often be detected in so called healthy individuals years before the full presentation of an autoimmune disease state. A 2006 study found that 20% of healthy Chinese subjects had one of three autoantibodies.19) Many researchers now espouse that early detection of these antibodies can help predict whether or not such a “healthy” person will develop an autoimmune disease. For example, in an eight-year prospective study, Swaak et al. examined the diagnostic significance of anti-double-stranded deoxyribonucleic acid (anti-dsDNA) determination in a group of 441 patients without systemic lupus erythematosus whose sera were found to contain antibodies to dsDNA on routine screening.20) Within one year, 69% (304) of these patients fulfilled the preliminary American Rheumatism Association (ARA) criteria for systemic lupus erythematosus (SLE). Eighty-two of the remaining 137 patients were followed up for several years. At the end of the study, 52% of these patients had also developed systemic lupus erythematosus. The team concluded that about 85% of patients without systemic lupus erythematosus with anti-dsDNA in the circulation would develop SLE within a few years.
Another recent study of blood from 441 healthy Portuguese blood-donors found autoantibodies for rheumatoid factor, anti cyclic citrunillated peptides, anti-mitochondria, anti-Sacharomyces cerevisiae, ANA, anti-TTG, and anti-Beta2- glycoprotein.21) More than 30% of the blood contained one or more of the antibodies, 4% exhibited two antibodies, and nearly 1% had three or more antibodies present. It is clear that sub-clinical autoimmune disease is much more common than previously thought.
This gradual presentation of an increasing number of so-called “autoantibodies” in the years before a patient meets the official criteria for an autoimmune diagnosis supports the model of successive infectionAn infectious cascade of pathogens in which initial infectious agents slow the immune response and make it easier for subsequent infections to proliferate. described earlier – pathogenic components of the microbiota gradually accumulate over the course of a lifetime until bacterial, viral and phage load reaches a level at which a diagnosis can be made. It also supports the contention that individuals perceived as “healthy” may still harbor and accumulate pathogenic microbes that will eventually lead to an inflammatory diagnosis, or a process associated with “aging.” Indeed, it is possible that any antibodies that damage “self” do so as an unintended polyspecific consequence of their activity against the metagenomic pathogens.
Other challenges to the theory of autoimmunity
Beyond the evidence showing that autoimmune patients are immunosuppressed, there are a number of other reasons why the autoimmune explanation of disease is problematic:
- The advent of novel molecular sequencing tools is showing how many pathogenic bacteria are missed using traditional techniques. It is no longer necessary to grow fastidious bacteria in vitro – that is, in a Petri dish – in order to prove they are present in the human body.
- A number of autoimmune disease have all the hallmarks of infection including granuloma, inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue., unique microbe populations, and co-infections.
- Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. patients' immunopathological response to low-dose antibiotics and a Vitamin D Receptor agonistA substance such as olmesartan (Benicar) or 1,25-D which activates the Vitamin D Receptor and transcribes the genes necessary for a proper innate immune response. is also highly suggestive of a bacterial pathogenesis.
- Organs and tissue from sarcoidosis patients have been shown to cause sarcoidosis in the transplanted recipients strongly suggesting that autoimmune diseases are transmissible. This is supported by the strong aggregation of chronic diseases in communities and families.
- Patients with autoimmune disease often show inconsistent signs of autoimmune reactivity:
The interesting thing was that sarcoidosis there are usually no antibodies. There is usually a very low sedimentation rate indicating that there's no real obvious pathogens, the immune system is not feeling anything. And that's one of the reasons it's such an idiopathic and has been such a puzzling disease.
Yet, when you look at the malaise suffered by the patients, the malaise is a straight line. There is no peaking of the malaise that was coincident with the peaking of the antibodies. In fact, the antibodies just seem to be bystanders, if you like, of the main disease process.
Trevor Marshall, PhD, 2006 Bio21 presentation
Evolutionary challenge
A number of evolutionary biologists have taken exception to the theory of autoimmunity based on what they know about how species change over time. According to evolutionary theory, there is no way that genes that determine a person will get an autoimmune disease will stay in a population for long. The forces of reproductive fitness are simply too strong.
One thing that we clearly know causes inflammation is the presence of an infection. So, as soon as I hear the word inflammation I think, “What infectious agents are at play?”
That brings us to the concept of autoimmune disease – the idea that the immune system just “goes crazy.” I think the fact that the concept of autoimmunity was developed in the first place is largely related to the fact that our brains have not evolved to think scientifically. People who have studied disease from their own point of view have recognized that the immune system is extremely important. But as we’ve learned more about the immune system, we’ve realized that it is an extremely complicated system - as complicated as the brain. Just like we can’t look at one type of neuron and infer information about the entire brain, we can’t try to understand the characteristics of only some immune cells and think we understand immune function.
So, over the years, as researchers have been daunted by the complexity of the immune system, it has seemed logical that such a complex entity has the potential to go wrong. Because they are limited by the power of their brains, they tend to simplify the issue and view the immune system in the same way they would view a truck that could break down. There are two problems with this type of thinking. For starters, we can’t trust our intuition that something complex is likely to malfunction. The fact is, the immune system functions just fine in a large proportion of the population. The only logical way to explain the immune activation seen in patients with “autoimmune disease” is to suggest that there is some sort of agent pushing the immune system off balance. This argument is only strengthened by the fact that the same evolutionary forces that would cause a serious disease to be weeded from the population would also cause those people whose immune systems are prone to self-destruction to be eliminated from the population.
The concept of autoimmune disease has progressed to the point that now even researchers who previously dismissed the possibility of infection are accepting the possibility that “autoimmune” disease could be triggered by infection. This is some progress, but it’s not enough. Especially since the concept of autoimmunity encourages doctors to prescribe immunosuppressive steroids to patients. But if persistent infection is involved these steroids may exacerbate the fire by allowing pathogens to spread.
Paul Ewald, Bacteriality.com
To think that autoimmune disease is caused by an interaction between the environment and human genetics, as a number of commentators have, is only marginally more plausible. Evolutionary theory is clear that any kind of consistent reproductive disadvantage is consistently and ultimately weeded out of the population.
Conventional therapies for autoimmunity
According to many doctors and researchers, the best way to treat an overreactive immune response is to suppress it. To that end, many patients with autoimmune diseases are given regular doses of medications that profoundly modulate a body's immune response including corticosteroidsA first-line treatment for a number of diseases. Corticosteroids work by slowing the innate immune response. This provides some patients with temporary symptom palliation but exacerbates the disease over the long-term by allowing chronic pathogens to proliferate., anti-TNF drugsDrugs which interfere with the body's production of TNF-alpha - a cytokine necessary for recovery from infection, and others. One provocative study concluded that reduced levels of vitamin D, a known immunosuppressant, was associated with autoimmune response in tuberculosis patients.22)
In some cases, patients may experience temporary symptom relief lasting months or even years. Yet, immunomodulatory drugs have a number of liabilities.
- range of negative side effects
- there is no proof of long-term survival benefit from use
- some drugs have been shown to cause relapse
- practice guidelines advocate moderate dosing
Also, if autoimmunity were causing disease, wouldn't disrupting molecular mechanisms put a stop to autoimmune disease? Shouldn't corticosteroids and anti-TNF drugs cure a patient of autoimmune disease?
Rethinking autoimmunity
Auto-antibodies, antibodies directed at the self, do exist and have been documented – but growing evidence suggests they don't cause autoimmune illnesses.
How then does one account for why these proteins are observed in patients with “autoimmune disease”? Is there a true target?
According to Trevor Marshall, autoantibodies are “incidental roadkill.” Intraphagocytic pathogens do damage via the innate immune system functions which they directly compromise, and auto-antibodies are a byproduct of the cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. and chemokine cascade which ensues.
It is quite plausible that “autoimmunity” is also caused by bacterial-induced alteration of human genes. All a bacterium would need to do in order to generate an apparent “autoimmune” reaction would be to interfere with the genes necessary for the production of proteins against which autoantibodies are produced.
According to one analysis, 463 human genes are changed during an infection with Mycobacterium tuberculosis.23) These mutated genes function in various cellular processes including intracellular signalling, cytoskeletal rearrangement, apoptosis, transcriptional regulation, cell surface receptors, cell-mediated immunity and cellular metabolic pathways.
There's no reason to think that other bacteria can do just as much damage – or that any pathogenic bacteria, for that matter, can interfere with production of autoantibodies.
[PMID: 22759465] [PMCID: 3472801] [DOI: 10.1159/000339149]
[PMID: 20813038] [PMCID: 2939603] [DOI: 10.1186/1465-9921-11-121]
[PMID: 21350705] [PMCID: 3040328] [DOI: 10.3748/wjg.v17.i5.567]
[PMID: 19948723] [PMCID: 2807280] [DOI: 10.1074/jbc.C109.071225]
[PMID: 19919305] [DOI: 10.1086/648408]
[PMID: 21353331] [DOI: 10.1016/j.jaad.2010.12.012]
[PMID: 17917546] [DOI: 10.1097/BOR.0b013e3282f0ad25]
[PMID: 3105056] [DOI: 10.1126/science.3105056]
[PMID: 214511] [PMCID: 2185048] [DOI: 10.1084/jem.148.5.1429]
[PMID: 9198668] [PMCID: 2196315] [DOI: 10.1084/jem.185.10.1721]
[PMID: 8898963] [DOI: 10.1002/eji.1830261031]
[PMID: 1299798] [DOI: 10.1111/j.1399-302x.1992.tb00630.x]
[PMID: 14007218] [PMCID: 290963] [DOI: 10.1172/JCI104523]
[PMID: 16963398]
[PMID: 17894023] [DOI: 10.1196/annals.1422.061]
[PMID: 16878294] [DOI: 10.1002/pmic.200500909]
[PMID: 3872637] [PMCID: 1001620] [DOI: 10.1136/ard.44.4.245]
[PMID: 22419776] [DOI: 10.1136/annrheumdis-2011-201127]
[PMID: 12890386]
[PMID: 8786337] [PMCID: 2192714] [DOI: 10.1084/jem.184.2.771]
[PMID: 14550878] [DOI: 10.1016/s1568-9972(03)00052-1]
[PMID: 20016507] [DOI: 10.1038/ni.1801]
[PMID: 16278064] [DOI: 10.1016/j.jaut.2005.09.024]
 … Medicine is so haphazard in its thinking…
… Medicine is so haphazard in its thinking…