Related article: Transmission of bacteria and onset of chronic disease

Table of Contents
Familial aggregation
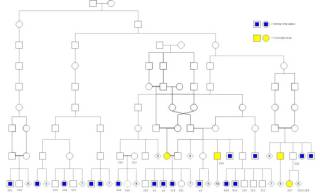
Familial aggregationOccurrence of a given trait shared by members of a family (or community) that cannot be readily accounted for by chance. refers to occurrence of a given trait shared by members of a family that cannot be readily accounted for by chance. For example we hear that certain diseases “run” in families, or we note that an entire family unit suffers from an inflammatory disease such as obesity. While it has long been understood that acute infections like tuberculosis, polio, and HIV are communicable, it is less well appreciated that the same can be said for the chronic pathogens which cause Th1 diseasesThe chronic inflammatory diseases caused by bacterial pathogens..
The reigning explanation for familial aggregation is that people pass down faulty genes to their offspring. However, the theory is not supported by solid evidence. Scientists have failed to find genes that might cause any major chronic inflammatory disease. In the case that they have found a relationship between a gene and a disease, statistical significance is usually so low that environmental influences such as bacteria could easily be causing the genetic mutations. To date, no form of gene therapy has proven effective for treating inflammatory disease.
Families
Growing evidence suggests that the Th1 pathogensThe community of bacterial pathogens which cause chronic inflammatory disease - one which almost certainly includes multiple species and bacterial forms., rather than faulty genes, are the driving factor behind familial aggregation.
Just like other forms of bacteria, the Th1 pathogens can be passed around. Although Th1 diseases are not obviously contagious, they are communicable – meaning that transmissionAn incident in which an infectious disease is transmitted. of chronic bacteria requires close contact and is seen often within the family unit. The pathogens can also be transmitted from person to person through bodily fluids released during coughing, sneezing and other intimate contact.
The Th1 pathogens gradually mutate genetic pathways and cause disease by a process known as successive infection.
Spouses develop the same diseases
Several studies have shown that spouses have a greater chance of developing the same disease as their partners - a phenomenon that can best be explained if familial aggregation has an infectious cause.
- sarcoidosis – A six-year study of the Th1 diseaseAny of the chronic inflammatory diseases caused by bacterial pathogens. sarcoidosis, conducted by the National Heart, Lung and Blood Institute at the National Institutes of Health (NIH) in Maryland found that among the 215 study participants who had been diagnosed with sarcoidosis, there were five husband-and-wife couples that both had the disease. Yet sarcoidosis is such a rare disease that, statistically speaking, there should have been none. This means the incidence of sarcoidosis in spouses in the study was 1,000 greater than could be expected by chance. The researchers also noted that the risk for sarcoidosis increased nearly five-fold in parents and siblings of people with the disease. 1)
- hypertension – Researchers at Queens Medical School in England found that men whose spouses had hypertension had a two-fold increased risk of hypertension. Similarly, women whose spouses had hypertension also doubled their risk of developing the disease. The risk for both male and female subjects persisted after adjustment for other variables such as diet. 2)
- exfoliative glaucoma – In the middle Norway eye-screening study, the prevalence of XFG in both members of married couples is significantly higher than expected, “thereby suggesting a common environmental (probably infectious) agent, which may be of aetiological significance for the XFG development.”3)
Family members develop the same diseases
Family members are much more likely to have a disease when a family member has the disease. This is especially true of diseases between mother and child: chronic diseases are often said to “pass down the maternal line.”
- autoimmune disease – A 2007 Danish study looked at the lifetime prevalence of 31 “autoimmuneA condition or disease thought to arise from an overactive immune response of the body against substances and tissues normally present in the body” diseases in 5.5 million people, the entire population of Denmark. According to the study's authors, there was “considerable familial aggregation in almost all 31 diseases.” In other words, if you have a sibling or parent with a disease, you are much more likely to have that disease yourself, in some cases more than one hundred times more likely. There were only three diseases for which there may have been some doubt and even those met the threshold of statistical significance.4)
- chronic fatigue syndrome – Dr. Garth Nicholson, a researcher at The Institute of Molecular Medicine in California has also conducted several studies on the communicability of diseases such as chronic fatigue syndrome, autism and Gulf War Syndrome (a disease with symptoms very similar to those of CFS). He noted that among soldiers who developed Gulf War Syndrome during the war in Iraq, 70% or more of family members showed symptoms of the same disease within 10 years after the soldier had returned from the war. At the 2006 Autoimmunity Research FoundationNon-profit foundation dedicated to exploring a pathogenesis and therapy for chronic disease. Conference in Chicago, British clinician and Chronic Fatigue Syndrome researcher Dr. Andy Wright stated that he very rarely sees a family in which the spouses do not both have the Th1 pathogens in their blood.
- autism – Scientists at the University of Maastricht in the Netherlands also found that relatives of individuals with autism often begin to show mild autistic traits, a phenomenon known as the broader autism phenotype (BAP). In one study conducted by the group, fathers with an autistic child demonstrated a different reaction time pattern and responded slower on the social cues than control fathers.5)
- Alzheimer's Disease – A 2010 NYU study using a PET scanner to examine the plaque in brains (which is the hallmark of Alzheimer's disease) found that a child's level of plaque was consistent with their fathers and especially their mothers – even years before a child had a diagnosis.6) The fact that amyloid-beta protein has recently been identified as an antimicrobial peptide7) suggests that what is being passed between the generations isn't so much the propensity to produce plaque, but the need to produce plaque in response to brain infection.
Family members are more likely to develop different diseases too
It should be noted that none of the above studies take into consideration the fact that spouses and siblings very often develop different forms of chronic disease. If researchers were to look for the incidence of Th1 disease among family members and take into account all possible inflammatory diagnoses, all of the above numbers would be notably higher.
- mental disorders – A 2008 study of parents of children with autism found they were more likely to have been hospitalized for a mental disorder than parents of control subjects. Schizophrenia was more common among case mothers and fathers compared with respective control parents.8) In a case study, Beleza-Meirles found that schizophrenia and mental retardation had very high rates of incidence in a particular family.9) A similar by Sveinbjörnsdóttir also observed high rates of Parkinson's in an extended family.10)
- autism and eye disorders – Researchers working at the University of Illinois at Chicago have found a striking trend: those with autistic relatives are more likely to show disrupted eye movement similar to their afflicted relation.11)
- autoimmune disease – In a study of families who have a member with primary Sjögren's syndrome, 38% had at least one first-degree relative with an autoimmune disease, versus 22% in control families.12) The most frequent autoimmune diseases registered among the patients' first-degree relatives were autoimmune thyroid disease, systemic lupus erythematosus, and rheumatoid arthritis.
As evidenced by progress reports on the Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. site, there are a substantial number of spouses who both suffer from chronic inflammatory diseases. There are also entire families on the MP - with each member using the treatment to eliminate his or her own pea-soup.
When one considers how often chronic diseases co-occur within a family unit – heart disease, arthritis, bipolar disorder, breast cancer, inflammatory bowel disease, Alzheimer’s disease – it becomes increasingly plausible that nearly all inflammatory diseases are communicable and that this communicability results in familial aggregation.
Non-relations in close proximity develop the same diseases
As would be expected for the Th1 diseases, which are transmitted via proximity and contact, even people who are not related pass diseases to each other.
- Alzheimer's – A subject whose spouse experienced incident dementia onset had a six times greater risk for incident dementia as subjects whose spouses were dementia free.13)
- multiple sclerosis – Multiple sclerosis occurred in epidemic form in various North Atlantic islands: probably in Iceland and the Shetland-Orkneys; clearly in the Faroe Islands. In the Faroes first symptom onset was in 1943, heralding the first of four successive epidemics at 13 year intervals. The disease was presumably introduced by occupying British troops during World War II, with the postwar occurrences representing later transmissions to and from consecutive cohorts of Faroese.14)
- obesity – Research suggests that obesity is also an inflammatory disease caused by certain species of the Th1 pathogens. A study recently published in the New England Journal of Medicine found that a person’s risk of becoming obese increases by 57% if they have a friend who becomes obese, and by 37% if their spouse becomes obese. 15) The researchers attributed the results to social factors, but the spread of bacteria is a more logical explanation.
- Parkinson's disease – A cluster of 13 cases of Parkinson's disease among a community of 592 people were reported in Israel, significantly more that would be expected by chance.16)
- sarcoidosis – A case-controlled study of residents of the Isle of Man found that 40 percent of people with sarcoidosis had been in contact with a person known to have the disease, compared with 1 to 2 percent of the control subjects. 17) Another study reported three cases of sarcoidosis among ten firefighters who apprenticed together. 18)
[PMID: 17684288] [DOI: 10.1513/pats.200607-138MS]
[PMID: 9830183] [PMCID: 1313221]
[PMID: 19854735] [DOI: 10.1136/bjo.2009.159046]
[PMID: 17582741] [PMCID: 2717015] [DOI: 10.1016/j.jaut.2007.05.002]
[PMID: 17588199] [DOI: 10.1007/s10803-007-0389-x]
[PMID: 20231448] [PMCID: 2851906] [DOI: 10.1073/pnas.0914141107]
[PMID: 20209079] [PMCID: 2831066] [DOI: 10.1371/journal.pone.0009505]
[PMID: 18450879] [DOI: 10.1542/peds.2007-2296]
[PMID: 18254962] [PMCID: 2259315] [DOI: 10.1186/1471-2350-9-6]
[PMID: 11114315] [DOI: 10.1056/NEJM200012143432404]
[PMID: 20679591] [PMCID: 3145411] [DOI: 10.1001/archgenpsychiatry.2010.87]
[PMID: 17086607]
[PMID: 10871801]
[PMID: 17652652] [DOI: 10.1056/NEJMsa066082]
[PMID: 2334236] [DOI: 10.1080/00039896.1990.9935931]
[PMID: 16844727] [PMCID: 2121155] [DOI: 10.1136/thx.2006.062836]
[PMID: 8214953] [DOI: 10.1164/ajrccm/148.4_Pt_1.974]