Related article: Managing dental and periodontal symptoms, Working with a dentist
Table of Contents
Periodontal disease and gingivitis
The tooth provides a surface for the colonization of a diverse array of bacterial species. Bacteria may attach to the tooth itself, to the epithelial surfaces (surface-lining tissue) of the gingiva or periodontal pocket, to underlying connective tissues, if exposed, and to other bacteria attached to these surfaces. In contrast to the outer surface of most parts of the body, the outer layers of the tooth do not shed, and thus microbial colonization (accumulation) is facilitated.1)
Periodontal disease is broadly classified as either gingivitis (inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue. of the gums) or periodontitis (inflammation of the ligaments and bones). Periodontitis is an ancient disease — fossil evidence demonstrates that our early ancestors experienced the localized alveolar bone loss around tooth root surfaces that is a hallmark of the disease. The loss of this bone, which supports the root structure of the tooth, leads to the eventual loss of the tooth.2)
While gingivitis is a reversible condition, affecting only the gingival tissue, it is a precursor to periodontitis. The transition from gingivitis to periodontitis may vary from weeks to years. Periodontitis is associated with attachment loss (teeth falling out) or alveolar bone loss.
Periodontal disease may be the most widely accepted example of successive infectionAn infectious cascade of pathogens in which initial infectious agents slow the immune response and make it easier for subsequent infections to proliferate. in that it involves multiples pathogens that accumulate over time. According to a widely held view:
Periodontal diseases are polymicrobial and reflect sequential colonization by a broad array of bacteria in the transition from a healthy subgingival biofilm A structured community of microorganisms encapsulated within a self-developed protective matrix and living together. to a diseased subgingival biofilm. While periodontal diseases are polymicrobial and reflect sequential colonization by a broad array of bacteria in the transition from a healthy subgingival biofilm to a diseased subgingival biofilm, the molecular mechanisms and synergism among the genera and species in the disease are not well understood.
Lakshmyya Kesavalu et al.3)
Types and prevalence
- periodontal disease – disease that affect one or more of the periodontal tissues; broadly classified as either gingivitis or periodontitis
- periodontitis – inflammation and infection of the ligaments and bones that support the teeth (alveolar bone)
- gingivitis – inflammation of the gums (gingiva); gingivitis always precedes periodontitis;4) characterized by gingival redness, swelling, and bleeding provoked by a periodontal probe, brushing, or flossing
Periodontitis is a major cause of tooth loss in adults. Almost 15 percent of the population in the United States (50 million) suffer from significant periodontal disease.5) A second study estimates that 116 million Americans suffer from periodontal disease.6) Moreover, approximately 50 percent of adults have gingivitis around more than six teeth.7) The prevalence of periodontal disease is closely associated with the level of oral hygiene.

Detecting bacteria
Related article: Detecting bacteria
Given its accessibility, the oral microbiome can easily be measured. Unlike, say, the kidney microbiome, institutional review boards rarely have reservations about researchers taking salivary samples. As a result, the microbial consortiums in dental plaque has become, according to a 2010 assessment, the most highly characterized in humans.8) This ease of detection may account for why evidence of the role of microbes in periodontal disease has been stronger than other diseases to date.
Culture-based studies
Culture-based studies have long identified substantial and diverse groups of bacteria living in the oral cavity. For example, in a 1971 analysis of the gingival crevice of healthy adults, the total microscopic counts averaged 2.7 x 1011 microorganisms per gram of wet weight.9) The total cultivatable anaerobic bacteria averaged 1.8 x 1011 microorganisms per gram, whereas facultative bacteria averaged 2.2 x 1010 microorganisms per gram, which is an eightfold difference. Overall, Streptococcus, Peptostreptococcus, Veillonella, Lactobacillus, Corynebacterium, and Actinomyces accounted for more than 80% of the total cultivatable oral flora.
Molecular analyses
However, results of surveys with molecular tools indicate a level of diversity in the human subgingival microflora that cannot be recognized by conventional culture techniques.10) More than 700 bacterial species from the oral cavity have been identified.11) In most instances, the cultivatable microflora probably represent less than 1% of the total extant population, as estimated by microscopy or other means.12)
In 2009, Nasidze et al. used 16S ribosomal RNA (rRNA) sequences from saliva samples from 120 healthy individuals (10 individuals from each of 12 worldwide locations).13) The sequences found could be assigned to 101 known bacterial genera, of which 39 were not previously reported from the human oral cavity. The team found there is high diversity in the salivary microbiome within and between individuals, but little geographic structure.
Evidence of infectious cause
Overwhelming data exist indicating that bacteria initiate the major mechanisms of destruction of the periodontium.14)
- Correlation between plaque and gingivitis – There is a positive correlation between the amount of bacterial plaque and the severity of gingivitis and amount of bone loss in cross-sectional studies of human populations.15)
- Antibody response to microbes – Numerous studies indicate that subjects with destructive periodontal diseases show an elevated serum antibody response to specific subgingival organisms.16)
- Pathogenic potential of plaque bacteria – Human bacterial plaque has demonstrated pathogenic potential when implanted into outside the mouth to sites such as subcutaneous destructive abscesses in humans or in experimental animals. A number of toxic products can be detected in dental plaque, including endotoxins, and cell wall mucopeptides. In addition, enzymes have been shown to be produced by whole plaque or individual microorganisms from plaque that can be demonstrated to hydrolyze a wide variety of tissue constituents. Finally, it must be pointed out that the bacterial masses that accumulate at or in the gingival sulcus possess an array of antigens and possibly polyclonal activators capable of triggering sequences of host-mediated events that have been postulated as mechanisms of tissue destruction.17)
- Experimental animal models – Studies in experimental animals have added considerable support to the hypothesis of the significant role of microorganisms in the etiology of periodontal disease. Germ-free animals are essentially free of periodontal destruction. Minimal observable inflammation or pocket formation and only minor. The use of antibiotics or antiseptics in a variety of animal model systems controls the soft tissue pathology and most of the hard tissue destruction in these animals. Periodontal disease can be transmitted from an animal harboring the disease to one initially free of it by caging diseased and disease-free animals together or by the implantation of plaque or specific microorganisms derived from the plaque of diseased animals. In addition, certain microorganisms isolated from human periodontal pockets can initiate periodontal destruction in several animal model systems.18)
Relationship with other diseases
Several researchers have noted a well-defined association between periodontal disease and gingivitis and other diseases.
Cardiovascular disease
Main article: Cardiovascular disease
One of the best known links between two different inflammatory diseases – and a prototypical illustration of successive infection – is the relationship between cardiovascular diseases such as heart attack and stroke, and periodontal disease. A BMJ paper showed a correlation between dental disease and systemic disease (stroke, heart disease, diabetes). After correcting for age, exercise, diet, smoking, weight, blood cholesterol level, alcohol use and health care, people who had periodontal disease had a significantly higher incidence of heart disease, stroke and premature death. More recently, these results were confirmed in studies in the United States, Canada, Great Britain, Sweden, and Germany. The magnitude of the association is striking: one study found that people with periodontal disease had a two times higher risk of dying from cardiovascular disease.19)
A 2010 study using pyrosequencing compared the bacterial diversity of atherosclerotic plaque, oral, and gut samples of 15 patients with atherosclerosis, and oral and gut samples of healthy controls.20) The team concluded that there was a high degree of correlation between the presence of certain bacteria in the oral cavity (and to a somewhat lesser extent the gut) with bacteria in the gastrointestinal tract. Interestingly, several bacterial taxa in the oral cavity and the gut correlated with plasma cholesterol levels. A 2011 study that compared heart attack victims to healthy volunteers found the heart patients had higher numbers of bacteria in their mouths.21) Their tests on 386 men and women who had suffered heart attacks and 840 people free of heart trouble showed two types – Tannerella forsynthesis and Prevotella intermedia – were more common among the heart attack patients. Interestingly, the total number of species that could be identified in the saliva was the best indicator that somebody was likely to have had a heart attack.
Successive infection dictates that as a person accumulates pathogens in one area of the body, those pathogens likely have mechanisms that allow them to slow the immune response. So, if people harbor greater number of pathogens in their mouths, the immune response may slow in the heart and arteries, making it easier for microbes to spread their as well. The same can be said for the opposite scenario. Also, it may be possible that microbes in the mouth spread toward the heart and arteries, although this has yet to be completely confirmed. However, some of the same bacteria identified in the salivary microbiome, such as Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis - both of which cause tooth decay22) - have also been identified in atherosclerotic plaque.23) 24)
Work by Kozarov et al. strongly suggests that “the key step [towards systemic infection] is the persistence of intracellular bacteria in phagocytes.” Bacterial strains, they conclude, “once in the circulation, are internalized by phagocytic cells, at which stage selected species avoid immediate killing and spread and colonize distant sites.”25) The group also showed that older patients, when compared to younger controls, have atheromas (plaques) which contain greater proportions of pathogens traditionally associated with periodontitis.26)
Our data demonstrate that the elderly individuals (mean age 67 years) have higher incidence of periodontopathogens in their plaques than the younger individuals…. Species from the Bacteroides family were found in about 17% of the young but in about 80% of the elderly patients, as expected given the association of this family with adult and refractory periodontitis.
Kozarov et al.27)
Given these similarities between the two disease states, along with the evidence presented above, it would be surprising if cardiovascular disease were not also definitively shown to be caused by the communities of microbes.
Other diseases
- Dementia – The immunosuppression caused by increased numbers of pathogens doesn't just affect the spread of microbes in the heart. For example, researchers at Vasant Hirani at University College London found that elderly people who have lost their teeth are at more than three-fold greater risk of memory problems and dementia.28)
- Diabetes – Poorly controlled diabetes may be a risk factor for increased severity of periodontitis and poor response to periodontal treatment. Some studies have shown that patients with poor control of diabetes and severe periodontitis show improvement in their A1c levels, as well as decrease in periodontal inflammation, with treatment of the periodontitis.29) 30)
- Pancreatic cancer – Particular types of mouth bacteria, some of which are found in gum disease, are associated with the development of pancreatic cancer, indicates a small study published online in the journal Gut.31) The authors base their findings on an initial comparison of the bacteria found in the spit of 10 patients with pancreatic cancer, which had not yet spread, and 10 healthy people, matched for age and sex. They found significant differences between the bacterial colonies in the two groups, with 31 additional species and 25 fewer species in the spit of the cancer patients. They then checked spit samples from a further 28 pancreatic cancer patients and 28 healthy people to verify their findings. And they checked tissue samples from 28 patients with chronic inflammation of the pancreas (chronic pancreatitis), which is associated with an increased risk of developing pancreatic cancer. Among six suspicious species, two – Neisseria elongata and Streptococcus mitis – showed up significantly less often in the mouths of the cancer patients than in those of their healthy peers, while levels of another species – Granulicatella adjacens – were significantly higher. The combination of N. elongata and S. mitis accurately differentiated between healthy patients and those with cancer in more than 80% cases. Furthermore, they found similar differences in the prevalence of S. mitis and G. adjacens between the chronic pancreatitis samples and the spit of healthy people.
- obesity – A 2011 cross-sectional study of obese adolescents versus normal weight subjects collected saliva performed a metagenomic analysis on that saliva. The study found that obese subjects had Campylobacter rectus and Neisseria mucosa in sixfold higher amounts than controls.[cite needed]32)
- joint failure – In a study of 36 patients with hip or knee joint failure, bacterial DNA from the synovial fluid was found to match microbial DNA from periodontal tissue in five patients.33)
Cause vs. effect
The correlations seen between periodontal disease and cardiovascular disease could be related to the transmissionAn incident in which an infectious disease is transmitted. of microbes from one infectious site to another. However, it seems more likely that when periodontal disease arises at the same time as other inflammatory conditions, these conditions are the result of the same underlying disease process. In this sense, periodontal disease does not necessarily cause cardiovascular disease, or vise versa. Rather, as the immune system in the entire body is weakened by an increasingly pathogenic microbiota, the body finds it more difficult to control microbial virulence in several body sites at once. Notably, Porphyromonas gingivalis, a periodontal pathogen associated with various forms of marginal periodontitis, is present in periodontal pockets undergoing destruction as well as in healthy gingival margins.34)
Successive infection
Related article: Successive infection
The microbiota associated with odontogenic infections are complex and generally reflect the indigenous oral flora. Such infections are typically polymicrobial, and invasiveness is often influenced by synergistic interactions of multiple microbial species.
Anthony W. Chow35)
Microorganisms were first considered as possible aetiological agents of periodontitis in the late 1800s, when the germ theory of disease changed our understanding of disease aetiologies. Failure to identify a specific pathogen in the polymicrobial community dampened the enthusiasm for a microbial aetiology, and other causes for perionditis, such as trauma or disuse atrophy, were proposed. Finally, however, the resolution of gingival inflammation after the physical removal of dental plaque during routine dental cleanings led to the “nonspecific plaque” hypothesis. The premise of this hypothesis is that the quantity of dental plaque is more important to disease pathogenesis than the identity of the individual bacterial species present.36)
Today, periodontal disease may be the most widely accepted example of successive infection, an infectious cascade of pathogens in which initial infectious agents slow the immune response and make it easier for subsequent infections to proliferate. Consistent with successive infection, no single bacterial species has been shown to be responsible for triggering the inflammatory host responses seen in periodontal disease.37) The sequential colonization by a broad array of bacteria in periodontal disease has been described by several commentators38) 39) 40) 41) 42) as well as Darveau:
Similarly to other polymicrobial diseases43) 44) 45), periodontitis has been characterized as a microbial-shift disease owing to a well-characterized shift in the microorganisms that are present (from mostly Gram-positive to mostly Gram-negative species46)) during the transition from periodontal health to periodontal disease.47)
Richard P. Darveau 48)
Three of the periodontal pathogens, Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, are commonly coisolated or identified in subgingival biofilm samples from adult periodontitis lesions.49) 50) 51) Increased numbers of several other bacterial species, including Campylobacter rectus, Eubacterium nodatum, Prevotella spp., Peptostreptococcus micros, and Streptococcus intermedius, as well as Fusobacterium nucleatum, have also been observed in deep periodontal pockets, and the presence of these organisms is positively correlated with increased probing depth and progressive periodontal ligament attachment loss 52) 53) 54) 55) These bacteria generally represent commensal opportunistic pathogens found at low levels at healthy sites; however, under the appropriate microenvironmental conditions, currently not well defined, they contribute to triggering periodontal disease progression.56) 57)
Synergistic activity of periodontal microbiota
In a 2007 study, groups of rats were infected with either Porphyromonas gingivalis, Treponema denticola, or Tannerella forsythia in monomicrobial infections or with all three species in polymicrobial oral infections with or without Fusobacterium nucleatum. Radiographic measurement of alveolar bone resorption showed that rats infected with the polymicrobial consortium with or without F. nucleatum exhibited significantly increased alveolar bone resorption compared to the resorption in uninfected control rats, as well as the resorption in rats infected with one of the microbes. Kesavalu et al. were able to conclude that these microbes acted synergystically resulting in the immunoinflammatory bone resorption characteristic of periodontitis.58)
Hematogenous seeding due to dental infection
One of the more straightforward examples of successive infection is the process by which infections of the teeth and gums (odontogenic infections) disseminate through the bloodstream to the body at large and “seed” native or prosthetic heart valves, joint replacements, or other prosthetic devices.59) For this reason, many physicians have recommended antibiotic prophylaxis as essential prior to any invasive dental procedure60) and routine examination of the oral cavity prior to elective prosthetic heart valve implantation or artificial joint replacement. It is precisely this type of infectious process, that the Marshall Pathogenesis points to as an early (and ongoing) driver of chronic disease.
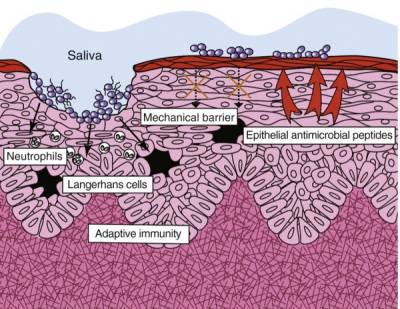
Periodontal microbes slow innate immune activity
In healthy periodontal tissue, all microbes, even commensal bacteria, elicit a robust innate immune responseThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease..62) 63) However, in tissue consistent with periodontal disease, innate immune activity is slowed by microbes. This is particularly well-documented in the pathogens known as the “red-complex organisms” – Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola – but virulence activity such as inhibition of IL-8 (see below) is present even in commensal microbes.64)
- Inhibition of the chemokine IL-8 – IL-8 is an important chemokine responsible for recruiting neutrophils to phagocytose the antigen, which in triggers the antigen pattern toll-like receptors such as TLR4. P. gingivalis, the best characterized periopathogen, can inhibit host defense functions — including the gingival epithelial secretion of IL-8 that is induced by other oral bacteria — by several mechanisms. One way is through production of phosphoserine phosphatase SerB, which contributes to the inhibition of IL-8. Synthesis of SerB is induced on contact with gingival epithelial cells and may modify normal host cell functions to create a suitable intracellular environment for the bacteria. This virulence mechanism, known as “local chemokine paralysis” impairs the production of IL-8 in clinically healthy tissue. Once the innate immune status of the periodontal pocket is compromised by the reduction in IL-8 secretion, neutrophil transit may be disrupted, resulting in an increase in the number and types of bacteria in the dental plaque.65)
- Modulation of signalling in “lipid rafts” – P. gingivalis has also been shown to interfere with an innate host defence protection mechanism by inducing crosstalk between TLR2A receptor which is expressed on the surface of certain cells and recognizes native or foreign substances and passes on appropriate signals to the cell and/or the nervous system. and CXC-chemokine receptor 4 (CXCR4) after they are recruited to a lipid raft in response to P. gingivalis fimbriae. This crosstalk attenuates the protective and bactericidal response to P. gingivalis. These findings provide another example of how bacteria manipulate the intricate regulatory mechanisms of host cells to survive in the host.66)
- Directly antagonizing TLR4 through production of Lipid A – The red-complex bacteria have been shown to also inhibit innate host defense by producing a lipid A structure that acts as a TLR4 antagonist. One experiment showed that a TLR4 antagonist decreased beta-defensin An antimicrobial peptide found primarily in immune cells and transcribed by the Vitamin D Receptor. expression, a key family of antimicrobial peptides.67)
The naturally occurring proteins in the human salivary proteins varies according to age.68)
Pathophysiology of alveolar bone and teeth resorption
Bone loss is complicated and multi-factorial.
- high levels of 1,25-D affect osteoclast activity – When functioning properly, the Vitamin D Receptor contributes to bone health in an underecognized way distinct from other classical calcium regulating hormones.69) However, proper functioning becomes disrupted when, according to the Marshall Pathogenesis, pathogenic microbes create proteins that bind and block the Vitamin D Receptor, preventing it from expressing many essential components required for health. These include the estrogen receptors, upon which the bone matrix is dependent. The bone matrix is dependent on estrogen homeostasis (estrogen is important to stimulate osteoblast activity).70) This model of disease is partially validated by the fact that drugs such as TNF-alpha inhibitors, which prevent inflammation, often lead to temporary increases in bone mass in patients with osteoporosis. Stimulated osteoclasts dissolve bone material, causing it to be reabsorbed into the bloodstream. This leads to bone loss as well as calcium being deposited in the soft tissues of the body, including those in the lungs, breasts and the kidneys (where it forms kidney stones).
- osteoporotic bone has lower levels of expression of VDR – A recent study evaluating expression of (rather than by) the VDR in platelets in osteoporotic bone versus healthy subjects.71) The authors found:
- lower levels of VDR in all patients,
- number of VDRs was positively correlated with bone density, and
- a decrease in the phosphorus levels in patients without differences in vitamin D levels and in the dietary calcium intake. The lower VDR expression in osteoporotic bone, the authors concluded, indicated a lower ability to respond to vitamin D, and could be the explanation of the increase in the PTH and decrease in the phosphorus levels in patients with respect to controls.
Microbes adhere to some root canal filling materials and sealers
Certain microorganisms have a high affinity to some root canal filling materials and sealers, especially to one named “gutta-percha.”72) Those microbes were Enterococcus faecalis, Streptococcus mutans, Streptococcus sanguis, and Prevotella nigrescens. Because of this high level of bacterial adhesion, subsequent biofilm formation on these materials could lead to the persistence of microorganisms in root canals.
"Healthy" bacteria play surprising role in gum disease
Developing gum disease may require not only bad bacteria but also a benign background microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms., researchers reported in Cell Host and Microbe.73) When the gingivitis-associated Porphyromonas gingivalis was introduced at low levels into the mouths of normal mice, it triggered a substantial growth in the healthy bacteria already there, and the ensuing periodontal disease led to bone loss.
But no such thing happened when the gingivitis bacteria were introduced to mice with sterile mouths that harbored no normal bacteria. It seems that a single species, even at low levels, can disrupt the stability of the bacterial ecosystem in the mouth, the researchers noted.
Recent research
In general, the mouth harbors at least six billion bacteria which are represented by more than 700 species (Theilade, 1990; Aas et al., 2005), as well as other types of microorganisms, including fungi, mycoplasma, protozoa, and possibly even viruses (Pennisi, 2005).
Despite the diverse community of oral microbiota, the oral cavity is, nonetheless, characterized by a stable community known as the climax community. Therefore, if imbalance in the oral resident microbiota occurs, oral diseases such as caries and periodontal diseases seem to appear, leading to multiplication of potentially pathogenic microorganisms.
While human subgingival plaque harbors more than 500 bacterial species, considerable research has shown that Porphyromonas gingivalis, a Gram-negative anaerobic bacterium, is the major etiologic agent which contributes to chronic periodontitis. This black-pigmented bacterium produces a myriad of virulence factors that cause destruction to periodontal tissues either directly or indirectly by modulating the host inflammatory response.
Various studies have shown that periodontitis occurs more often among patients with systemic diseases such as diabetes mellitus, AIDS, leukemia, and Down’s syndrome. 74)
Oil pulling is a traditional folk remedy practiced in ancient India. It is believed to cure more than thirty systemic diseases when practiced regularly and as directed. 75)
Patients experiences
I just returned from my astonished dental hygienist, she spent a considerable amount of time looking for documented problems, with no success! She was baffled, could the improvement in my general health be so profound that it could be seen in my oral health, also?! Absolutely!
Sue Lyons, MarshallProtocol.com
The recessed area of my gums have in fact filled in now. Nice!
Cynthia Schnitz, MarshallProtocol.com
I use baking soda for brushing and follow with dilute hydrogen peroxide as a mouth wash….
I have been adjusting my view on innate immune system assistance to include the mouthwash regimen. It is simple, low tech, and not expensive. In a way, one could almost put daily dental care into the category of good first aide, and for people who have or have borderline periodontal disease, it is first aide care.
I really consider this topic essential for an MPr's expedited recovery. Thanks for opening my eyes on its importance (hat tip to Marion for her insights also). I find that I am not always the quickest off the mark and slow to see things, so I really appreciate the help here.
Mvanwink, MarshallProtocol.com
I've had dental problems since childhood, and as an adult, ~15 emergency root canals, jawbone infections, and more. It seems that many here have had inexplicable dental woes over the years (well, now we know why!) that are getting better on the MP.
Alayne, MarshallProtocol.com
The dentist commented how clean and plaque-free my teeth were. I told him it must be the MP, as there's no change in the way I treat them
Julia, MarshallProtocol.com
Pre-MP I had constant build up of tartar on my teeth - it's now gone - haven't had any for months.
PaulT, MarshallProtocol.com
Prior and early on while on the MP, I had major periodontal disease and had been recommended to have extensive surgery. I asked to have any procedures delayed for 6 months. On re-evaluation, I had only two small pockets which were treated with laser surgery. My gums have remained healthy since and regression apparent prior has now resolved back to normal. Also, my calculus or tartar build-up is much, much less.
…
In fact, I had a problematic tooth, constant low grade swelling which all dentists I saw said could be an issue but to wait and see. After being in phase 2 for a couple of months during which it would get painful for periods, it now has no swelling and my gums have been in the best condition in my life….
Also, I no longer produce tartar at the rate I did previously. I am confident that many of these chronically infected root canal teeth will not need to be extracted after adequate treatment of the Th1 condition and that any that are truly a problem will blow up and obviously be extracted at that time.
Greg Blaney, M.D., MarshallProtocol.com
All of my life my teeth and gums have been an issue for me. I think every tooth in my head has a root canal and a crown. When I was 20, I started to have gum problems and needed extensive curretage by a periodontist – expensive, time consuming and not fun. I could probably pay off the national debt if I had the money my teeth and gums have cost me.
My gums were under control at the start of the MP, but there were several areas that were borderline and needed constant attention: special toothbrush, water pic, dental floss and many trips to the dentist but I still had one area that was inflamed and never seemed to clear up.
Was I surprised that six or so months into the protocol all of a sudden I realized my gums were the healthiest they have ever been in my life. I now have no pockets, my receding gum line has become “unreceded” – no more bleeding gums, they have never been so healthy. This pointed out to me how powerful the MP is and that it really is killing bacteria in areas I didn't even know I had any.
Aunt Diana, MarshallProtocol.com
My dentist also told me that my gums are improving.
Sharon, MarshallProtocol.com
On the MP for 3 months. My pinkish-red, puffy gums are returning to a nice shade of light pink again and my gum recession is diminishing.
Elisabeth, MarshallProtocol.com
I asked Matt a few days ago how his gums were doing as I hadn't heard him complain about them for a while. Beginning September 06 the gums on one side bled each time he cleaned his teeth and they felt a bit swollen. Sensitive toothpaste and an anti-inflammatory / anti-bacterial mouth gel helped ease Matt's gums. It is called “Difflam mouth gel” and contained the cetylpyridium chloride that Trevor recommended (1 mg/g) (was all I could get). Matt applied it 3 times per day, not recommended for use for more than 7 days- helped a lot and after a few weeks the problem subsided. Matt says his gums still feel a bit swollen but they don't bleed any more and they are not sore. He just seems to ignore it now. Will be interesting to see if that particular immunopathologyA temporary increase in disease symptoms experienced by Marshall Protocol patients that results from the release of cytokines and endotoxins as disease-causing bacteria are killed. comes back.
Robyn O., Mom of Matt, MarshallProtocol.com
Very early during MP I had jaw herxs, which resulted in regaining my cheek bones and dimples profile.I can chew stronger and longer and never have bleeding gums when cleaning my teeth.
Grace, MarshallProtocol.com
My dentist said what's left of my gums are in great shape and he is astounded how quickly that turned around (Now if ONLY I had gotten on the MP before I allowed them to cut away most of the gums around lower molars.
I'm no expert here but in my opinion your gums can and will heal on the MP. I couldn't tell you how much. I, like you had a lot of gum issues about a year before my official diagnosis of sarcoidosis. I had 3-4 fructations, receding gum tissue and bone loss. Scalings, etc. didn't do any good and my dentist sent me to a periodontist to have gum surgery. This was about the time I got diagnosed with sarcoidosis and I really felt it all had to be related (I probably had it for many years but about 6-7 months of trying to get fitter and taking lots of omega 3 via fish oil and flax seed oil really kicked off the sarcoidosis ….now I know it was due to high vit d).
I asked the periodontist about it… he was convinced periodontal disease and did the surgery. He did however take a biopsy of some of the diseased gum tissue and low and behold, sarcoidosis. He sent me to a Oral Surgeon that he knew for a consult and I was told that periodontal disease would look exactly like sarcoidosis to a periodontist (and vice-versa). At this point (about 4 years ago) I was told I would lose 4-5 molars over the next several years.
Interestingly enough, after starting the MP, the gum tissue's have gotten healthy (the little that remains :( ) and have stabilized. I have 1 tooth in particular that was very mobile…it still is mobile but less so than before (of course, a lot of the root is sticking out so not much remains).
If I had it all to do over again (hindsight is 20/20), I'd try 6 months on the MP before I let someone cut away on my gums….I might very well have had a lot less tissue removed. I know due to the gum loss I'd already experienced that it would not have returned completely well…but I bet I would have a lot more than I have now.
…
I too, like your husband have had very bad issues with what was diagnosed as periodontal disease. This was right at the same time as my diagnosis for Sarc, imagine that. I ended up having gum surgery which I wish I wouldn't have had…but that was before I got on the MP. The gum surgery resulted in a biopsy that showed oral sarcoidosis (and an oral surgeon told me that to someone not looking for Sarc as I had told my dentist about it ahead of time, it would have just looked like gum disease). As it turns out, shortly after gum surgery, I convinced my Rheumy at the time to NOT do another round or prednisone, but treat me with at least minocycline (since he wouldn't go for the benicar part of the MP). My results were that the minocycline by itself really helped my gum issues. I suspect if I had just gone on minocycline, without the treatment, it would have improved the chance to save my gums. However, to totally cure the TH1 disease in the gum tissue, I believe one has to go on the MP.
My Rheumy had me do 100MG 2X per day…after figuring out that i needed to “pulse” the mino, I did so at 100 mg QOD. That actually started me herxing and convinced me that the MP was the way to go. Another 16 months and I finally switched docs and joined the MP (18 months ago). Too bad for my poor gums….I will lose at least 4 teeth where the gums were cut away so much the exposed roots are as large as the tooth themselves.
Robertrr, MarshallProtocol.com
Two months in phase 2. Good news from the periodontist who told me yesterday when I went for my 6 monthly gum clean that my gums are the best they have been since I had the surgery for gum disease nearly 6 years ago. I was quite surprised as I had not been as diligent as I usually am in their maintenance over the last 6 months. I must say the cleaning process wasn’t nearly as horrible as it usually is and my gums were not tender and bleeding afterwards. Thank you Trevor!
McAleesa, MarshallProtocol.com
For the first time in more than 10 years I have perfect gums, no pockets - this cleared up in the last 6 months. My dentist and hygienist were shocked. They've rarely seen such dramatic improvement in such a short time. My dentist has a friend with CFS and she went home to check your website the same day she was so impressed.
Sandiegojoy, MarshallProtocol.com
I have just returned from a periodonist appointment. Lasttime he told me my gums had improved incredibly. This time he couldn't find any evidence of infection. I had surgery approx 6 years ago for gum disease. It has been a constant struggle since then to keep the infection under control. I was warned that I would eventually lose two teeth….. Well I was told today that if my gums remain at this level I will not lose my teeth. Great hey!!! I had a good talk to the periodentist and asked him if he had lots of patients with so called auto immune diseases. He affirmed this. I told him gum disease was a symptom of these disorders and as he showed interest told him to check the website. I hope he does. Go Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis..
Scarab (Alex), MarshallProtocol.com
[PMID: 9673160] [DOI: 10.1111/j.1600-0757.1994.tb00016.x]
[PMID: 20514045] [DOI: 10.1038/nrmicro2337]
[PMID: 17210663] [PMCID: 1865722] [DOI: 10.1128/IAI.00733-06]
[PMID: 765622]
[PMID: 2066519] [DOI: 10.1016/s0002-8177(91)26016-x]
[PMID: 10965470] [DOI: 10.1111/j.1752-7325.1999.tb03237.x]
[PMID: 9526927] [DOI: 10.1902/jop.1998.69.2.269]
[PMID: 4935491] [PMCID: 377340] [DOI: 10.1128/am.21.6.1046-1050.1971]
[PMID: 10588742] [PMCID: 24473] [DOI: 10.1073/pnas.96.25.14547]
[PMID: 11371542] [PMCID: 95255] [DOI: 10.1128/JB.183.12.3770-3783.2001]
[PMID: 12620860] [PMCID: 150096] [DOI: 10.1128/AEM.69.3.1687-1694.2003]
[PMID: 19251737] [PMCID: 2665782] [DOI: 10.1101/gr.084616.108]
[PMID: 10197286] [DOI: 10.1177/204748739900600102]
[PMID: 20937873] [PMCID: 3063583] [DOI: 10.1073/pnas.1011383107]
[PMID: 21375559] [DOI: 10.1111/j.1600-0528.2010.00582.x]
[PMID: 10699021] [PMCID: 86374] [DOI: 10.1128/JCM.38.3.1196-1199.2000]
[PMID: 15662025] [DOI: 10.1161/01.ATV.0000155018.67835.1a]
[PMID: 17981690] [DOI: 10.2741/2822]
[PMID: 20972353] [DOI: 10.5551/jat.5207]
[PMID: 16513386] [DOI: 10.1016/j.micinf.2005.09.004]
[PMID: 17767683] [DOI: 10.1111/j.1532-5415.2007.01298.x]
[PMID: 11314885] [DOI: 10.1034/j.1600-051x.2001.028004306.x]
[PMID: 15766369] [DOI: 10.1111/j.1600-051X.2005.00658.x]
[PMID: 21994333] [PMCID: 3705763] [DOI: 10.1136/gutjnl-2011-300784]
[PMID: 22426587] [PMCID: 3888235] [DOI: 10.1097/RHU.0b013e3182500c95]
[PMID: 20712638] [DOI: 10.1111/j.1600-0757.2010.00352.x]
[PMID: 14530302] [DOI: 10.1177/154411130301400504]
[PMID: 15330940] [DOI: 10.1111/j.1600-0757.2004.03671.x]
[PMID: 15853940] [DOI: 10.1111/j.1600-0757.2005.00107.x]
[PMID: 9495612] [DOI: 10.1111/j.1600-051x.1998.tb02419.x]
[PMID: 17699621] [PMCID: 1959459] [DOI: 10.1073/pnas.0706625104]
[PMID: 18487399] [PMCID: 2519371] [DOI: 10.1128/AEM.02884-07]
[PMID: 19394334] [PMCID: 2963147] [DOI: 10.1053/j.gastro.2009.04.046]
[PMID: 7865085] [DOI: 10.1177/08959374940080022001]
[PMID: 15853935] [DOI: 10.1111/j.1600-0757.2005.00108.x]
[PMID: 11018133] [DOI: 10.1146/annurev.micro.54.1.413]
[PMID: 9345222]
[PMID: 16297740] [DOI: 10.1016/j.idc.2005.07.002]
[PMID: 19161412] [DOI: 10.1111/j.1600-065X.2008.00701.x]
[PMID: 12960260] [DOI: 10.1189/jlb.0203082]
[PMID: 19675119] [DOI: 10.1177/1753425909104899]
[PMID: 19591489] [DOI: 10.1021/pr900212u]
[PMID: 20813899] [DOI: 10.1182/blood-2010-04-279216]
[PMID: 12682916] [DOI: 10.1002/jcb.10486]
[PMID: 22801440]
[PMID: 21846541] [DOI: 10.1016/j.joen.2011.05.034]
[PMID: 22036469] [PMCID: 3221781] [DOI: 10.1016/j.chom.2011.10.006]
[PMID: 26903954] [PMCID: 4746253] [DOI: 10.3389/fmicb.2016.00053]
[PMID: 28053895] [PMCID: 5198813] [DOI: 10.1016/j.jtcme.2016.05.004]