Related article: Innate immune response and Th1 inflammation

Table of Contents
Metabolism of vitamin D and the Vitamin D Receptor
A number of studies have suggested that patients with chronic inflammatory diseases are deficient in 25-hydroxyvitamin-DThe vitamin D metabolite widely (and erroneously) considered best indicator of vitamin D "deficiency." Inactivates the Vitamin D Nuclear Receptor. Produced by hydroxylation of vitamin D3 in the liver. (25-D) and that consuming greater quantities of vitamin D, which elevates 25-D levels, alleviates symptoms of disease. Some years ago, molecular biology identified 25-D as a secosteroid. Secosteroids would typically be expected to depress inflammation, which is in line with the reports of symptomatic improvement. The simplistic first-order mass-action model used to guide the early vitamin studies has given way to a more complex description of action. When active, the Vitamin D nuclear receptor (VDR) affects transcription of at least 913 genes and impacts processes ranging from calcium metabolism to expression of key antimicrobial peptides.
Located in the nucleus of a variety of cells including immune cells, the VDR is a control system of sorts. When exposed to infection and damage, especially that which is caused by pathogens, the body begins to convert the inactive form 25-D into the active form, 1,25-D. As cellular concentrations of 1,25-D increase, 1,25-D activates the VDR, turning on any number of genes the receptor transcribes.
Hormonal changes result from change in 1,25 dihydroxyvitamin-D
According to a 2010 analysis, the VDR significantly affects 229 human genes. Many of these genes have long been associated with autoimmune diseases and cancers including, for example, the genes IRF8 (linked to multiple sclerosis), and PTPN2 (connected to Crohn's disease and type I diabetes).1) The activation of certain genes also leads to the synthesis of antimicrobial peptides. The antimicrobial peptides are the body's “natural antibiotics” and have a potent anti-bacterial effect.
However, bacteria create ligands, which like 25-D, inactivate the VDR and, in turn, the innate immune responseThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease.. This allows the microbes to proliferate. In response, the body increases production of 1,25-D from 25-D, leading to one of the hallmarks of chronic inflammatory disease: a low 25-D and a high 1,25-D.
This pattern is a result of the disease process rather than a cause. For a variety of reasons, neither increased consumption of vitamin D nor the body's synthesis of additional 1,25-D is ultimately effective at combatting infection.
Vitamin D Receptor (VDR) controls innate immunity
Related articles: Metabolism of vitamin D and the Vitamin D Receptor , Aiming at health
Regulating the VDR
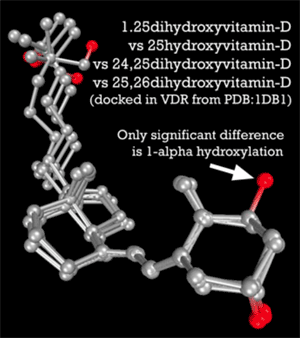
A general appreciation for how 25-D and 1,25-D compete for nuclear receptors gets to the heart of their opposing roles in the body. According to the Marshall Pathogenesis, the VDR is foremost a control system. Under most circumstances, the active form, 1,25-D, acts as the “on” switch and the inactive form, 25-D, is the “off” switch.2) 25-D is not completely inactive, but it does not and cannot activate the VDR. As Leow states in Respirology, “25-D levels are not associated with levels of cathelicidin Family of antimicrobial peptides found primarily in immune cells and transcribed by the Vitamin D Receptor. or beta-defensin An antimicrobial peptide found primarily in immune cells and transcribed by the Vitamin D Receptor.-2 [antimicrobial proteins transcribed by the VDR].”3)
Further underscoring this role for these two D metabolites is that 25-D and 1,25-D “happen” to share similar binding affinities for the VDR. According to molecular modeling by Trevor Marshall, PhD, 1,25-D has an affinity of 8.48 (as measured by nanomolar Kd) and 25-D has an affinity of 8.36.4) It would seem that activation of the Vitamin D nuclear receptor is achieved by a delicate balance between the concentrations of a number of endogenous hormones. Indeed, at the risk of overgeneralization, the body increases and decreases the production of 1,25-D to control the innate immune response.
As mentioned before, exposure to injury and infection enhances production of 1,25-D, which in turn leads to the creation of antimicrobial peptides and activation of TLR2.
However, certain feedback mechanisms are also in place, which allow the body to limit the production of 1,25-D to just that amount needed for proper transcriptional activation of the VDR.
- When the VDR is activated, it transcribes the gene for the enzyme CYP24A1, which increases conversion of 1,25-D into inactive metabolites.
- An activated VDR also controls 1,25-D concentration by limiting transcription of the gene CYP27B1, which converts 25-D into 1,25-D.5)
Bacteria and the VDR
The Antimicrobial Peptide Database lists hundreds of antimicrobial peptides known to kill or inhibit the reproduction of bacteria,6) 793 AmPs found in animals as of January 5, 2009. The sheer diversity of these proteins coupled with the fact that they have been conserved over millenia suggests that enough pathogenic bacteria exist in sufficient quantities to warrant the evolution of these defense mechanisms. It would seem that is in the strong interest of the human body to destroy or disrupt these bacteria.
Pathogenic bacteria are likewise driven by evolutionary impetus: it's in their interest to disrupt the proteins, which interfere with their growth. In what way or ways could bacteria interrupt production of the AmPs?
According to one researcher, it is nearly impossible for bacteria to develop resistance to the AmPs:
Acquisition of resistance by a sensitive microbial strain against antimicrobial peptides is surprisingly improbable.
Michael Zasloff 7)
However, what if it were possible to disrupt the expression of the Vitamin D Receptor by secreting ligands, which bind to and inactivate the receptor? Such bacteria would have an undeniable reproductive advantage.
Think about this for a minute – if you were a persistent pathogen, wouldn’t it seem a good idea to disable your host’s ability to produce antimicrobial peptides? And if you discovered that disabling just one receptor, the VDR, would get rid of both cathelicidin and defB2, wouldn’t you try to evolve a mechanism for doing that?
Trevor Marshall, PhD
Bacteria disable the VDR
In the arms race of host–microbe co-evolution, successful microbial pathogens have evolved ingenious ways to evade host immune responses.8)
Studies have indicated that the dysregulation of VDR may lead to exaggerated inflammatory responses, raising the possibility that defects in Vitamin D and VDR signaling transduction may be linked to bacterial infection and chronic inflammation. Further characterization of Vitamin D/VDR will help elucidate the pathogenesis of various human diseases and in the design of new approaches for prevention and treatment.
Jun Sun 9)
Since the VDR is at the heart of the innate immune system, bacteria can survive by discovering how to disable it through a variety of different actions. Actions accumulate and are more powerful than individual actions.
In keeping with evolutionary theory, a growing number of substances and species have been shown to downregulate the activity of the VDR:
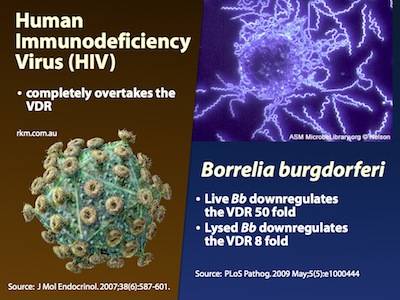
- Borrelia burgdorferi – Live Borrelia burgdorferi reduced VDR expression in monocytes (phagocytes) by 50 times, and lysates (“dead” Borrelia) reduced it by 8 times10)
- Mycobacterium tuberculosis enzyme involved in vitamin D and 7-dehydrocholesterol metabolism 14)
- Mycobacterium leprase – produces microRNA-21 to target multiple genes associated with the VDR15)
- “Gliding” biofilm A structured community of microorganisms encapsulated within a self-developed protective matrix and living together. bacteria have been shown to create Capnine – Capnine is a 2-amino-3-hydroxy-15-methylhexadecane-1-sulfonic acid, and is created by the genera Cytophaga, Capnocytophaga, Sporocytophaga, and Flexibacter.16) 17) The secretion of capnine meets an important evolutionary need for bacteria. Capnine possesses a high affinity for the VDR as evidenced by the fact that molecular modeling shows that its stable in the ligand binding pocket. Molecular modeling further shows that when capnine is docked in the VDR, it inactivates the receptor.
- Chlamydia trachomatis – shown to downregulate the VDR in unpublished work
The following substances reduce the number of VDR, without which immune function is limited:
According to the Marshall Pathogenesis, pathogens' production of ligands, which bind to and antagonize (inactivate) the Vitamin D Receptor, is one of the fundamental processes by which chronic inflammatory disease occurs. The consumption of other immunosuppressive substances also has an effect.
One promising area for future research is to fully characterize the breadth and diversity of proteins created by bacteria in infected cells.
Murine models of VDR offer some confirmation
One can see the effects of a dysfunctional VDR in knockout mice, mice genetically engineered to be born without the receptor. These mice demonstrate what it is like to have a VDR completely blocked by bacterial ligands.
Mice without a VDR have been shown in separate studies to be born with alopecia, an inflammatory condition in which organisms have no hair20) and age prematurely.21) Scientists have also found that Salmonella is much more virulent and aggressive in mice in which the vitamin D receptor had been turned off.22) These mice showed higher levels of activity of inflammatory molecules, and they lost weight more quickly and were much more likely to die in response to infection.
Further validating the Marshall Pathogenesis model is this: other research in VDR knockout mice has shown a marked increase, by a factor of ten, in serum 1,25-D and a clear reduction - to almost undetectable levels - in serum 25-D. Such levels persisted at seven weeks until the mice eventually died.23)
Mechanisms by which bacteria affect levels of 25-D and 1,25-D
A chronic pathogenic microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms. affects the levels of the D metabolites observed in chronic diseases in several ways. When the immune system is challenged by pathogens, the body activates CYP27B1, causing more 25-DThe vitamin D metabolite widely (and erroneously) considered best indicator of vitamin D "deficiency." Inactivates the Vitamin D Nuclear Receptor. Produced by hydroxylation of vitamin D3 in the liver. to be converted to 1,25-DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol., which, of course, increases activity of the VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response..
However, just because the concentration of 1,25-D reaches high levels - sometimes extremely high values - does not mean that the hormone is successful in binding and activating all of the body's Vitamin D Receptors (VDR). In fact, a 1,25-D that is elevated for an extended period of time suggests that the activity of the VDR is at least partially blocked.
When bacterial ligands block the VDR, the Receptor is prevented from transcribing CYP24A1, a well-studied enzyme which breaks down excess 1,25-D.
A full understanding of all these mechanisms supports the conclusion that elevated 1,25-D and depressed 25-D are a result rather than a cause of the inflammatory disease process.
Viruses and fungi also affect the VDR
- Epstein-Barr virus (EBV) – shown to downregulate expression of the VDR (and by the VDR) by a factor of about five, inducing “eventual immortalization”24) A 2011 paper further showed that a EBV not only down-regulates expression of the VDR protein itself, but also acts to block transcription by the VDR.25)
- Aspergillus fumigatus – In cystic fibrosis patients, the fungus A. fumigatus has been shown to secrete gliotoxin, a toxin which dose-dependently downregulates VDR mRNA and protein levels. This directly results in decreased levels of the AmP LL-37, thereby “providing an opportunistic environment for both bacterial and fungal colonization.”28) 29)
- Cytomegalovirus – Cytomegalovirus affects hundreds of genes including downregulating the VDR 2.2 fold.30)
- Hepatitis C virus – Gal-Tanamy et al. showed that HCV infections interfere with the VDR by inhibiting the creation of CYP24A1, the enzyme responsible for breaking down excess 1,25-D.31)
Evidence for high 1,25-D in patients with chronic disease
Under most normal conditions, serum levels of 1,25-dihydroxyvitamin D are constant throughout the year (no variability due to sun exposure), but there is no such tight biochemical regulation in at least some chronic inflammatory diseases such as obesity.32)
At the site of infection
It is sometimes thought that the liver and kidney are the only sites for conversion of 25-D into 1,25-D, but there is evidence that this process happens outside those organs – not coincidentally, at the very sites where patients report symptoms of chronic disease. High levels of 1,25-D and the enzyme which leads to the production of 1,25-D, 1 α-hydroxylase, have been found at various locations where the human body needs a strong host defense.33)
- skin cells of sarcoidosis patients – Sarcoidosis patients have a variety of skin symptoms including bumps, ulcers, or discolored skin. Zehnder et al found increased expression of the enzyme 1 α-hydroxylase – the enzyme which converts 25-D into 1,25-D – in the skin cells of sarcoidosis patients.34) They write:
In particular, the expression of 1α-OHase [1 α-hydroxylase] by activated macrophages and epidermal keratinocytes [skin cells] suggests a role for 1,25(OH)2D3 [1,25-D] as an immunomodulatory and/or antiproliferative hormone.
- synovial fluid surrounding the joints of patients with rheumatoid arthritis – Mawer et al found that 1,25-D levels were particularly elevated in the synovial fluid surrounding the joints of patients with rheumatoid arthritis (RA).35) In this study, median serum levels of 1,25-D at baseline was not elevated in the RA patients — only 24 pg/ml. Thus, the extrarenal synthesis of 1,25-D was not obvious from the routine blood test for 1,25-D. There is no reason to think that the metabolism of other diseases is any different.
- immune cells including macrophages – Research has also shown that 1,25-D is synthesized in cells of the immune system, including the T cells and antigen-presenting cells36) as well as the macrophages.37) 38) The fact that the immune cells are a site for 1,25-D synthesis is notable, because it is these cell types, especially macrophages, which are often infected by the Th1 pathogens.
There is strong evidence that the extrarenal enzyme located in macrophages plays a major role in certain granulomatous conditions (e.g., sarcoidosis), causing uncontrolled elevations of blood 1,25-(OH)2D3 levels….
Glenville et al.39)
- respiratory epithelial cells – The primary lung epithelial cells, which play an important role in the defense against airborne pathogens, have been shown to express high baseline levels of activating 1 α-hydroxylase and low levels of inactivating 24-hydroxylase. This activity leads to increased expression of genes by the Vitamin D Receptor.40)
These disease site-specific peaks in 1,25-D somewhat undercuts the validity of the 1,25-D serum blood test as the gold standard for measuring the presence of chronic disease. There is currently no clinically available whole body test for elevated 1,25-D. For this reason, the ultimate measure of diseases treatable by the Marshall Protocol is the therapeutic probeA brief trial of the Marshall Protocol to see if it will generate an immunopathological response. The "gold standard" for testing whether a patient is a good candidate for the MP..
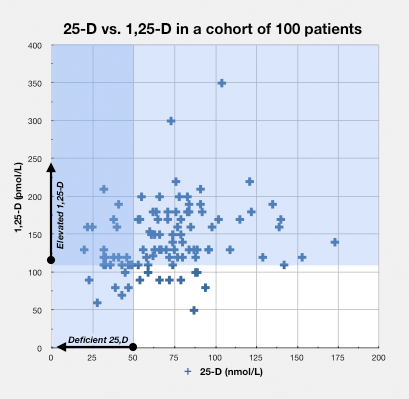
In the blood serum
As 1,25-D increases, it sometimes leaks into the bloodstream where it can be detected by measures of the metabolite in blood serum, but certainly not always.
A number of studies have demonstrated that the level of the hormone 1,25-D rises in patients with certain chronic diseases.
- autoimmune disease – Greg Blaney MD, a physician who practices in Vancouver, British Columbia (a setting with relatively infrequent sunlight), found that of a group of 100 patients with a variety of chronic inflammatory diseases, 85 had elevated measures of 1,25-D, which was defined as greater than or equal to 110 pmol/L.41)
- Crohn's disease – One study found that in a cohort of 88 Crohn's disease patients, 35 patients or 40% had elevated levels of 1,25-D, which the authors defined as above 60 pg/mL.42)
- obesity – Moan et al. showed that, in contrast to healthy people, patients with a high body mass index (BMI) had a significant seasonal variation in, not only 25(OH) vitamin D, but also of 1,25-D.43)
- sarcoidosis – Kavathia et al found that in patients with sarcoidosis, those with high serum levels of 1,25-D have more pronounced chronic treatment needs.44)
- asthma – Liu et al. showed that levels of vitamin D metabolites, particularly 1,25-D, were low within the airways and increased after allergen challenge. The increases correlated with the magnitude of inflammation and increases in cathelicidin.45)
Bell listed the following diseases and conditions, which manifest with high levels of 1,25-D: tuberculosis, AIDS with Pneumocystis carinii pneumonia, AIDS with cytomegalovirus infection, disseminated candidiasis, leprosy, rheumatoid arthritis, silicone-induced granulomas, Wegerner’s granulomatosis, Hodgkin’s disease, lymphoma, histocytic lymphoma, T-cell leukemia, plasma cell granuloma, leiomyoblastoma, seminoma, and subcutaneous fat necrosis.46)
Effect of high 1,25-D on nuclear receptors
In our study, the response to 1,25(OH)2D3 appears to be biphasic with a stimulatory effect at lower concentrations, and becoming inhibitory or ineffective at the higher levels.
Saveria Aquila et al.47)
At normal levels, the active vitamin D metabolite, 1,25-D, serves an important role in host defense,48) but high levels of the hormone are immunosuppressive49) – if for no other reason than the fact that it is calcitriol (1,25-D) and its analogues are used widely to treat autoimmune disease. One of the mechanisms by which 1,25-D may be immunosuppressive (and contribute to symptoms of disease) is by interacting with the body's other nuclear receptors. Selvaraj et al. have suggested that the high levels of 1,25-D seen in patients with pulmonary tuberculosis “might lead to downregulation of VDR expression” and that “decreased VDR levels could result in defective VDR signaling.”50) More recently, Johan Lundqvist's 2011 study showed that, consistent with the 2009 study by Proal et al. that “1α,25-dihydroxyvitamin D3 exerts tissue-specific effects on estrogen and androgen production and metabolism.”51)
Molecular modeling data show that at high levels, 1,25-D not only binds the VDR but also has a strong affinity for other key receptors that control the body's major hormonal systems including those that regulate the body's sex, thyroid, and adrenal hormones. As 1,25-D rises, it pushes out the molecules that are meant to control these receptors. Compromising these receptors can disrupt the body's ability to regulate temperature, libido, and any number of other functions.[table of affinities needed] Indeed, in the human brain, the VDR tends to be most common in the hypothalamus, which is responsible for these functions.52)
Molecular research also shows that, like the VDR, several of these nuclear receptors (including the alpha/beta thyroid receptors, glucocorticoid receptor, and androgen receptor) also express many families of antimicrobial peptides. A recent analysis of AmP expression by Brahmachary showed that the glucocorticoid receptor, the androgen receptor, and the Vitamin D Receptor, seem to be in control of 20, 17 and 16 families respectively, out of 22 analyzed.53) This means that when elevated 1,25-D displaces their endogenous ligands, the body's overall AmP expression is thwarted to an even greater degree. This further impairs the innate immune system's ability to combat chronic pathogens.
Case in point: thyroid receptor
Related article: Presentation - Vitamin D induced dysregulation of nuclear receptors may account for higher prevalence of some autoimmune diseases in women
1,25-D has a very high affinity for the alpha thyroid nuclear receptorIntracellular receptor proteins that bind to hydrophobic signal molecules (such as steroid and thyroid hormones) or intracellular metabolites and are thus activated to bind to specific DNA sequences which affects transcription. (ThRa) having a Kd value of 8.41. Normally levels of the endogenous ligand for ThRa known as T3 (which has a Kd 7.20 for ThRa) keep 1,25-D out of the binding pocket, but as 1,25-D rises due to VDR dysregulation it starts to proportionately displace T3 and block transcription by ThRa. The same thing should happen with thyroid beta – 1,25-D has a Kd of 8.44 for that receptor.
When 1,25-D displaces T3, the genes with ThRa promoters are no longer transcribed, resulting in the phenomenon known as thyroid hormone resistance. Since related nuclear receptors work as a group, when transcription by ThRa is dysregulated, system wide gene transcription is also affected.
Case in point: androgen receptor
1,25-D has a kD of 8.05 for the androgen receptor, and a Kd of 8.12 for the glucocorticoid receptor. Elevated 1,25-D can displace cortisol and testosterone from their target receptors as well, leading to an array of other hormonal imbalances.
Effect of high 1,25-D on TACO
A primary action of 1,25-D is that a high level in susceptible individuals (e.g. during pregnancy and sun holidays) causes the cellular membrane protein TACO to allow tiny bacteria to freely enter and exit the immune cells, without causing the cells to die in the process.
Dr. Andy Wright has taken photos of [bacteria freely entering and exiting cells], and it obviously would allow the pathogen(s) to spread without restraint.
Trevor Marshall, PhD
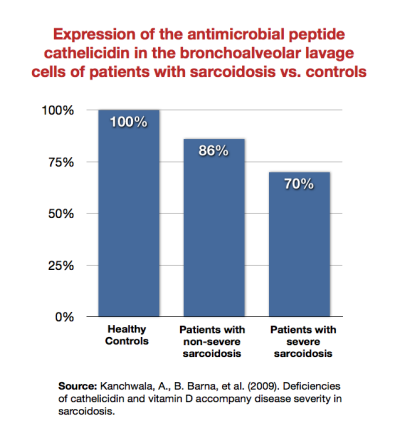
End-stage disease
As patients become sicker, their immune system becomes increasingly unable to mount a defense against infection. For example, Kanchwala et al. showed that patients with sarcoidosis expressed the antimicrobial peptide cathelicidin less than healthy subjects, and that sicker sarcoidosis patients expressed it least of all.54)
One of the hallmarks of late-stage inflammatory diseases is a very low 1,25-D with HIV/AIDS being the most commonly cited example.
HIV/AIDS
Related article: HIV and AIDS
HIV is a viral infection, and AIDS is the syndrome, which results – according to the Marshall Pathogenesis – in a dysregulated vitamin D metabolism. As evidenced by the subset of people who survive for decades with HIV, the virus itself is not necessarily deadly. Instead, it is the co-infections which are the proximate cause of the disease. One can define the breadth of AIDS-related complications by the extent and number of co-infections such as pneumonia, herpes, Candida, etc.
Supporting this hypothesis, a number of terminal AIDS patients have neglible levels of 1,25-D. 18 of 29 patients in a study of AIDS patients had undetectable levels of the metabolite.55) The patients with depressed levels of 1,25-D were characterized by advanced clinical HIV infection, low CD4+ lymphocyte counts, and high serum levels of tumor TNF-alpha – all indication of more severe forms of the disease.
The exact mechanism by which 1,25-D is downregulated is not entirely known, but it is highly likely that it is caused by pathogens.
Haug et al theorized that TNF-alpha and possibly other cytokinesAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. – which pathogens are known to create56) – inhibit conversion of 25-D into 1,25-D in late-stage cases of HIV/AIDS.57)
A second factor is that HIV disables D-Binding protein (DBP).58) 59) DBP is the precursor for Macrophage Activating Factor (MAF) as it has a key role to play in attracting macrophages to sites of injury. 1,25-D is transported throughout the blood by DBP.
Cancer
Related article: Vitamin D and cancer
Higher levels of CYP24A1, the enzyme which breaks down excess 1,25-D, is associated with poorer survival in lung adenocarcinoma. In a 2011 study, CYP24A1 mRNA was elevated 8-50 fold in lung cancer (compared to normal non-cancerous lung) and significantly higher in less severe cancers.60)
Lopes et al. showed that CYP24A1 expression was increased in metastatic breast cancer: 53.7% in invasive carcinomas compared to 19.0% of benign lesions.
From this study, we conclude that there is a deregulation of the Vitamin D signalling and metabolic pathways in breast cancer, favouring tumour progression. Thus, during mammary malignant transformation, tumour cells lose their ability to synthesize the active form of Vitamin D and respond to VDR-mediated Vitamin D effects, while increasing their ability to degrade this hormone.
N. Lopes,61)
Related publications and presentations
Read more research
[PMID: 20736230] [PMCID: 2945184] [DOI: 10.1101/gr.107920.110]
[PMID: 18200565] [DOI: 10.1002/bies.20708]
[PMID: 21244571] [DOI: 10.1111/j.1440-1843.2011.01924.x]
[PMID: 17368181] [DOI: 10.1016/j.jsbmb.2006.12.038]
[PMID: 18957441] [PMCID: 2686604] [DOI: 10.1093/nar/gkn823]
[PMID: 11807545] [DOI: 10.1038/415389a]
[PMID: 21350579] [PMCID: 3077082] [DOI: 10.1038/nri2918]
[PMID: 20639756] [PMCID: 2955835] [DOI: 10.1097/MOG.0b013e32833d4b9f]
[PMID: 19461888] [PMCID: 2679197] [DOI: 10.1371/journal.ppat.1000444]
[PMID: 12890386]
[PMID: 11461902] [DOI: 10.1074/jbc.M102876200]
[PMID: 9784538] [PMCID: 108664] [DOI: 10.1128/IAI.66.11.5314-5321.1998]
[PMID: 27289046] [DOI: 10.1016/j.jsbmb.2016.05.021]
[PMID: 22286305] [PMCID: 3274599] [DOI: 10.1038/nm.2584]
[PMID: 2992489] [DOI: 10.1016/0006-291x(85)90497-8]
[PMID: 6330048] [PMCID: 215589] [DOI: 10.1128/jb.159.1.42-46.1984]
[PMID: 18832097] [PMCID: 2646540] [DOI: 10.1210/en.2008-1217]
[PMID: 17696608] [PMCID: 1941748] [DOI: 10.1371/journal.ppat.0030111]
[PMID: 17517646] [PMCID: 1890511] [DOI: 10.1073/pnas.0702884104]
[PMID: 19500727] [DOI: 10.1016/j.jsbmb.2009.03.007]
[PMID: 9241280] [DOI: 10.1038/ng0897-391]
[PMID: 19550398]
[PMID: 20593215] [DOI: 10.1007/s00018-010-0441-4]
[PMID: 9814454] [DOI: 10.1210/jcem.83.11.5270]
[PMID: 19209727] [PMCID: 3263379]
[PMID: 22904183] [DOI: 10.1164/rccm.201203-0478OC]
[PMID: 18566437] [PMCID: 2614917] [DOI: 10.4049/jimmunol.181.1.698]
[PMID: 21793032] [DOI: 10.1002/hep.24575]
[PMID: 19444938] [DOI: 10.1016/j.jsbmb.2009.01.001]
[PMID: 18981129] [PMCID: 2596683] [DOI: 10.4049/jimmunol.181.10.7090]
[PMID: 11158062] [DOI: 10.1210/jcem.86.2.7220]
[PMID: 1950677] [DOI: 10.1002/jbmr.5650060711]
[PMID: 14696037] [DOI: 10.1002/bies.10368]
[PMID: 12887108]
[PMID: 9790574] [DOI: 10.1152/physrev.1998.78.4.1193]
[PMID: 19758177] [DOI: 10.1111/j.1749-6632.2009.04875.x]
[PMID: 15247180] [PMCID: 1774134] [DOI: 10.1136/gut.2003.036657]
[PMID: 20071158] [PMCID: 4778713] [DOI: 10.1016/j.rmed.2009.12.004]
[PMID: 22092530] [DOI: 10.1111/j.1365-2222.2011.03879.x]
[PMID: 9525334] [DOI: 10.1359/jbmr.1998.13.3.350]
[PMID: 19948036] [PMCID: 2794269] [DOI: 10.1186/1477-7827-7-140]
[PMID: 19943126] [DOI: 10.1007/s12185-009-0452-9]
[PMID: 19758324] [DOI: 10.1111/j.1600-0625.2009.00955.x]
[PMID: 19219539] [DOI: 10.1007/s10875-009-9277-9]
[PMID: 21262387] [DOI: 10.1016/j.bbalip.2011.01.004]
[PMID: 15589699] [DOI: 10.1016/j.jchemneu.2004.08.006]
[PMID: 17254313] [PMCID: 1764486] [DOI: 10.1186/1471-2105-7-S5-S8]
[PMID: 16466631] [DOI: 10.5483/bmbrep.2006.39.1.001]
[PMID: 16545013] [DOI: 10.1089/aid.2006.22.262]
[PMID: 19031451] [DOI: 10.1002/jmv.21376]
[PMID: 21169243] [PMCID: 3058389] [DOI: 10.1158/1078-0432.CCR-10-1789]
[PMID: 20831823] [PMCID: 2945944] [DOI: 10.1186/1471-2407-10-483]