Main article: Pulsed low-dose antibiotics

Table of Contents
Biofilm bacteria
Biofilms A structured community of microorganisms encapsulated within a self-developed protective matrix and living together. are densely packed communities of microbial cells that grow on living or inert surfaces and surround themselves with secreted polymers. Many bacterial species form biofilms, and their study has revealed them to be complex and diverse. The structural and physiological complexity of biofilms has led to the idea that they are coordinated and cooperative groups, analogous to multicellular organisms.1)
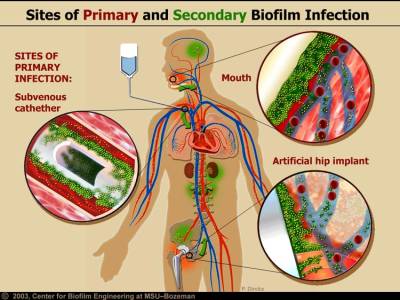
Researchers have estimated that 60-80 percent of microbial infections in the body are caused by bacteria growing as a biofilm A structured community of microorganisms encapsulated within a self-developed protective matrix and living together. – as opposed to planktonic (free-floating) bacteria.
There is a perception that single-celled organisms are asocial, but that is misguided. When bacteria are under stress—which is the story of their lives—they team up and form this collective called a biofilm. If you look at naturally occurring biofilms, they have very complicated architecture. They are like cities with channels for nutrients to go in and waste to go out.
Andre Levchenko, PhD, Johns Hopkins University
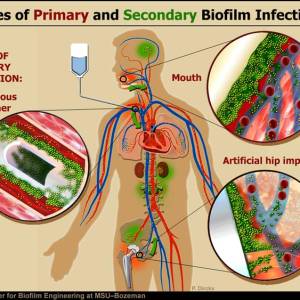
Some external biofilm, namely chronic wounds and dental plaque, can be manually removed. Because of their inaccessibility and heightened resistance to certain antibiotic combinations and dosages, internal biofilm are more difficult to eradicate.
Biofilm bacteria are a part of what is known as the Th1 bacterial pathogens, which according to the Marshall PathogenesisA description for how chronic inflammatory diseases originate and develop., collectively cause chronic disease. The Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. targets the Th1 pathogensThe community of bacterial pathogens which cause chronic inflammatory disease - one which almost certainly includes multiple species and bacterial forms., in part, through the use of pulsed low doses of antibiotics, because they limit the growth of “persister cells.”
Biofilm-related disease. 2)
History of biofilm research
Perhaps because many biofilms are thick enough to be visible to the naked eye, the microbial communities were among the first to be studied by early microbiologists. Anton van Leeuwenhoek scraped the plaque biofilm from his teeth and observed what he described as the “animalculi” inside them under his primitive microscope.
In the years which followed, researchers have concentrated primarily on planktonic (free-floating) bacteria, the kinds of microbes studied by the likes of Louis Pasteur and Robert KochAuthor of Koch's postulates, a set of rules for establishing a relationship between a causative microbe and a disease. Koch's belief that only one pathogens causes one disease has now been called into question as multiple postulates are increasingly considered out of date.. It was not until the 1970s that scientists began to appreciate that bacteria in the biofilm mode of existence constitute such a major component of the bacterial biomass in most environments. In the 1980s and 1990s, scientists began to understand how elaborately organized a bacterial biofilm community can be.3)
Paul Stoodley of the Center for Biofilm Engineering at Montana State University, attributes much of the lag in studying biofilms to the difficulties of working with heterogeneous biofilms compared with homogeneous planktonic populations. In a 2004 paper in Nature Reviews, the molecular biologist describes many reasons why biofilms are extremely difficult to culture, such as the fact that the diffusion of liquid through a biofilm and the fluid forces acting on a biofilm must be carefully calculated if it is to be cultured correctly. According to Stoodley, the need to master such difficult laboratory techniques has deterred many scientists from attempting to work with biofilms.4)
Although research on biofilms has surged in the last 20-30 years, the majority of biofilm research to date has focused on external biofilms, or those that form on various surfaces in our natural environment. Better tools to analyze external biofilms has realized they cause a wide range of problems in industrial environments. For example, biofilms can develop on the interiors of pipes, which can lead to clogging and corrosion. Biofilms on floors and counters can make sanitation difficult in food preparation areas.
Since biofilms have the ability to clog pipes, watersheds, storage areas, and contaminate food products, large companies with facilities that are negatively impacted by their presence have naturally taken an interest in supporting biofilm research, particularly research that specifies how biofilms can be eliminated.
This means that many recent advances in biofilm detection have resulted from collaborations between microbial ecologists, environmental engineers, and mathematicians. This research has generated new analytical tools that help scientists identify biofilms.
Prevalence of biofilm
According to a recent public statement from the National Institutes of Health, more than 65% of all microbial infections are caused by biofilms…. If one recalls that such common infections as urinary tract infections (caused by E. coli and other pathogens), catheter infections (caused by Staphylococcus aureus and other gram-positive pathogens), child middle-ear infections (caused by Haemophilus influenzae, for example), common dental plaque formation, and gingivitis, all of which are caused by biofilms, are hard to treat or frequently relapsing, this figure appears realistic.
Kim Lewis 5)
Diseases for which biofilm have been implicated
In just a short period of time, researchers studying internal biofilms have already determined they cause a number of chronic infections and diseases. Notable diseases include:
- atherosclerosis – Biofilm may contribute to the development of atherosclerosis. Ott et al.'s work showed a diverse groups of bacterial “signatures” in atherosclerotic lesions of patients with coronary heart disease.6) In a commentary following Ott's paper, Katz and Shannon concluded that his work suggested that atherosclerotic plaques are composed of “functional biofilm.” The team noted that the characteristics of a “mature” arterial wall make it well-suited for biofilm formation and explains the inefficacy of antibiotics, such as macrolides or fluoroquinolones, in clinical trials.7)
- chronic sinusitis – One study found that biofilms are present on the removed tissue of two-thirds of patients undergoing surgery for chronic inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue. of the sinuses.8)
- chronic wounds – Biofilm have been implicated in chronic wounds. Dr. Randall Wolcott has published work offering strategies for managing wounds.9)
Molecular analyses of chronic wound specimens revealed diverse polymicrobial communities and the presence of bacteria, including strictly anaerobic bacteria, not revealed by culture. Bacterial biofilm prevalence in specimens from chronic wounds relative to acute wounds observed in this study provides evidence that biofilms may be abundant in chronic wounds.
GA James et al. 10)
- cystic fibrosis – The lungs of individuals with cystic fibrosis are colonized and infected by bacteria from an early age. These bacteria, which often spread amongst individuals with CF, thrive in the altered mucus, which collects in the small airways of the lungs. Over time, both the types of bacteria and their individual characteristics change in individuals with CF. In the initial stage, common bacteria such as Staphylococcus aureus and Hemophilus influenzae colonize and infect the lungs. Eventually, however, Pseudomonas aeruginosa (and sometimes Burkholderia cepacia) dominates. Once within the lungs, these bacteria adapt to the environment and develop resistance to commonly used antibiotics. Pseudomonas can develop special characteristics that allow the formation of large colonies, known as “mucoid” Pseudomonas, which are rarely seen in people that do not have cystic fibrosis.11) Infection by the bacterium Pseudomonas aeruginosa is the main cause of morbidity and mortality among patients with cystic fibrosis.12)
- endocarditis – Inflammation of the smooth membranes which line the inside of the heart is caused by a complex biofilm composed of both bacterial and host components.13)
- inner ear infections – The majority of ear infections are caused by biofilm bacteria.14) These infections, which can be either acute or chronic, are referred to collectively as otitis media (OM). They are the most common illness for which children visit a physician, receive antibiotics, or undergo surgery in the United States.
It appears that in many cases recurrent disease stems not from re-infection as was previously thought and which forms the basis for conventional treatment, but from a persistent biofilm…. [The discovery of biofilms in the setting of chronic otitis media represents] a landmark evolution in the medical community’s understanding about a disease that afflicts millions of children world-wide each year and further endorses the emerging biofilm paradigm of chronic infectious disease.
Garth Ehrlich, PhD
- kidney stones – Biofilms also cause the formation of kidney stones.15) The stones cause symptoms of disease by obstructing urine flow and by producing inflammation and recurrent infection that can lead to kidney failure. Approximately 15%–20% of kidney stones occur in the setting of urinary tract infection. According to Matthew Parsek, PhD these stones are produced by the interplay between infecting bacteria and mineral substrates derived from the urine. This interaction results in a complex biofilm composed of bacteria, bacterial exoproducts, and mineralized stone material.
- leptospirosis – Biofilms also cause leptospirosis, a serious but neglected emerging disease that infects humans through contaminated water. Previously, scientists believed the bacteria associated with leptospirosis were planktonic (free-floating). One research team has shown that Leptospira interrogans can make biofilms, which could be one of the main factors controlling survival and disease transmissionAn incident in which an infectious disease is transmitted..16) According to the study's author, 90% of the species of Leptospira tested could form biofilms, and it takes L. interrogans an average of 20 days to make a biofilm.
- osteomyelitis – According to Parsek, biofilms may also cause osteomyelitis, a disease in which the bones and bone marrow become infected. This is supported by the fact that microscopy studies have shown biofilm formation on infected bone surfaces from humans and experimental animal models.17)
- osteonecrosis and osteomyelitis of the jaw – Of 20 patients with these bone disease, all “exhibited large surface areas of bone occluded with well-developed biofilms.”18)
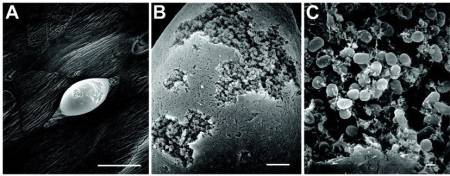
- periodontal disease – Perhaps the most well-known and studied biofilm bacteria. Hundreds of microbial biofilm colonize the human mouth, causing tooth decay and gum disease.
Plaque is a biofilm on the surfaces of the teeth. This accumulation of microorganisms subject the teeth and gingival tissues to high concentrations of bacterial metabolites which results in dental disease.
Matthew Parsek, PhD 19)
Dental plaque is composed of more than 500 species.20)
- prosthetic joints and heart valves – Pathogenic biofims are also commonly found on medical devices such as joint prostheses and heart valves.21) Dr. Patel of the Mayo Clinic has concluded that prosthetic joints increase the likelihood of biofilm infection.
When people think of infection, they may think of fever or pus coming out of a wound. However, this is not the case with prosthetic joint infection. Patients will often experience pain, but not other symptoms usually associated with infection. Often what happens is that the bacteria that cause infection on prosthetic joints are the same as bacteria that live harmlessly on our skin. However, on a prosthetic joint they can stick, grow and cause problems over the long term. Many of these bacteria would not infect the joint were it not for the prosthesis.
Robin Patel, MD, EurekaAlert!
- urinary tract infections – In their 2003 Science paper, Anderson et al. reported that in the case of UTIs, intracellular Escherichia coli can mature into biofilms, creating pod-like bulges on the bladder surface. explains how bladder infections can persist in the face of robust host defenses.22) “The idea that biofilms might form inside human cells is really novel,” said internist Pradeep Singh of the University of Iowa College of Medicine in Iowa City, who studies lung biofilms that plague children with cystic fibrosis.
- veterinary diseases – Biofilms have also been implicated in a wide array of veterinary diseases.23)
Drinking water
According to a 2011 review, biofilms in drinking water systems can serve as a significant environmental reservoir for pathogenic microorganisms.24)
Life cycle of biofilm communities
Attachment/colonization
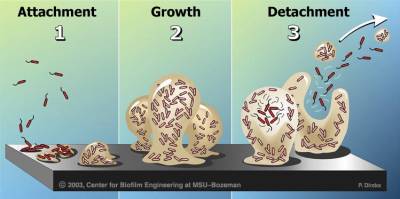
Biofilms form when bacteria adhere to surfaces in aqueous environments and begin to excrete a slimy, glue-like substance that can anchor them to a variety of materials including metals, plastics, soil particles, medical implant materials and, most significantly, human or animal tissue. The first bacterial colonists to adhere to a surface initially do so by inducing weak, reversible bonds called van der Waals forces. If the colonists are not immediately separated from the surface, they can anchor themselves more permanently using cell adhesion molecules, proteins on their surfaces that bind other cells in a process called cell adhesion.
These bacterial pioneers facilitate the arrival of other pathogens by providing more diverse adhesion sites. They also begin to build the matrix that holds the biofilm together. If there are species that are unable to attach to a surface on their own, they are often able to anchor themselves to the matrix or directly to earlier colonists. The expression of 800 genes have been shown to be altered when a single bacterial species joins a biofilm.25)
According to Costerton, the genes that allow a biofilm to develop are activated after enough cells attach to a solid surface.
It appears that attachment itself is what stimulates synthesis of the extracellular matrix in which the sessile bacteria are embedded. This notion– that bacteria have a sense of touch that enables detection of a surface and the expression of specific genes– is in itself an exciting area of research.
William Costerton et al. 26)
Research on the molecular and genetic basis of biofilm development has shown that when cells switch from planktonic to community mode, they also undergo a shift in behavior that involves alterations in the activity of numerous genes. There is evidence that specific genes must be transcribed during the attachment phase of biofilm development. In many cases, the activation of these genes is required for synthesis of the extracellular matrix that protects the pathogens inside.
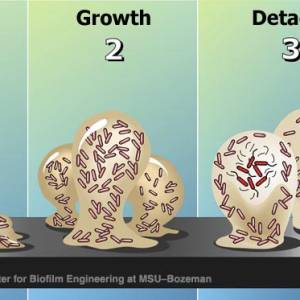
Growth and development
After the initial colonization, the biofilm grows through a combination of cell division and recruitment. The next stage of biofilm formation is known as development and is the stage in which the biofilm is established and may only change in shape and size.
Once a biofilm has more fully formed, it often contains channels in which nutrients can circulate. Cells in different regions of a biofilm also exhibit different patterns of gene expression. Because biofilms often develop their own metabolism, they are sometimes compared to the tissues of higher organisms, in which closely packed cells work together and create a network in which minerals can flow.
Biofilms grow slowly, in diverse locations, and biofilm infections are often slow to produce overt symptoms.
Movement
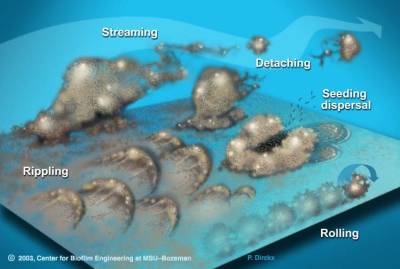
Biofilm bacteria can move in numerous ways that allow them to easily infect new tissues. Biofilms may move collectively, by rippling or rolling across the surface, or by detaching in clumps. Sometimes, in a dispersal strategy referred to as “swarming/seeding”, a biofilm colony differentiates to form an outer “wall” of stationary bacteria, while the inner region of the biofilm “liquefies”, allowing planktonic cells to “swim” out of the biofilm and leave behind a hollow mound.27)
Detachment and external colonization
Researchers often note that, once biofilms are established, planktonic bacteria may periodically leave the biofilm on their own. When they do, they can rapidly multiply and disperse. There is a natural pattern of programmed detachment of planktonic cells from biofilms. This means that biofilms can act as what Costerton refers to as “niduses” of acute infection. Because the bacteria in a biofilm are protected by a matrix, the host immune system is less likely to mount a response to their presence.28)
But if planktonic bacteria are periodically released from the biofilms, each time single bacterial forms enter the tissues, the immune system suddenly becomes aware of their presence. It may proceed to mount an inflammatory response that leads to heightened disease symptoms. Thus, the periodic release of planktonic bacteria from some biofilms may be what causes many chronic relapsing infections.
As Matthew R. Parsek of Northwestern University describes in a 2003 paper in the Annual Review of Microbiology, any pathogen that survives in a chronic form benefits by keeping the host alive.29) After all, if a chronic bacterial form simply kills its host, it will no longer have a place to live. So according to Parsek, chronic infection often results in a “disease stalemate” where bacteria of moderate virulence are somewhat contained by the defenses of the host. The infectious agents never actually kill the host, but the host is never able to fully kill the invading pathogens either.
Parsek believes that the optimal way for bacteria to survive under such circumstances is in a biofilm, stating that “Increasing evidence suggests that the biofilm mode of growth may play a key role in both of these adaptations. Biofilm growth increases the resistance of bacteria to killing and may make organisms less conspicuous to the immune system…. ultimately this moderation of virulence may serve the bacteria’s interest by increasing the longevity of the host.”
Advantages of biofilm
Biofilm communities provide several advantages to their members including easy access to food and nutrients and resistance to antibiotics.
Antibiotic resistance
This development of a biofilm allows for the cells inside to become more resistant to the body's natural antimicrobials as well as the antibiotics administered in a standard fashion. In fact, depending on the organism and type of antimicrobial and experimental system, biofilm bacteria can be up to a thousand times more resistant to antimicrobial stress than free-swimming bacteria of the same species.
Ability to enter into latent states during inhospitable conditions
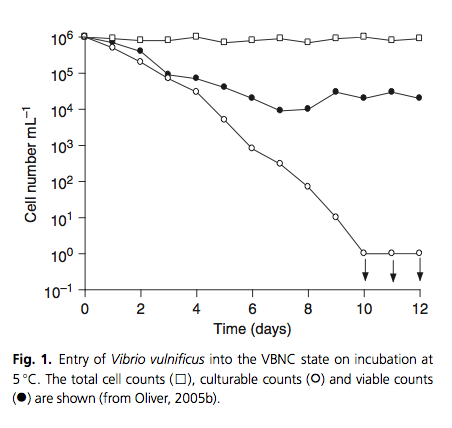
In the midst of inhospitable conditions such as nutrient starvation, microbes in biofilm communities can enter into a viable but nonculturable state.30) According to Epstein,31) members of microbial communities periodically wake up from this state of dormancy. In a method analogous to “sending out scouts” to “test the environment” for its suitability for growth of the entire population. In this scenario, if the resuscitating cells “detect” that the previously stressful/adverse environment is now growth-permissive, they would signal the remaining cells to resuscitate.32)
Freeloaders
Even though a biofilm tends to benefit all its members, understanding how such cooperation among pathogens evolves and is maintained may represent one of evolutionary biology’s thorniest problems. Biofilm bacteria appear to resolve the problem of freeloaders in at least two ways:
- Increasing species diversity – Once inside a biofilm, P. fluorescens, for example, differentiates into various forms, each of which uses different nutrient resources. The fact that these “diverse cooperators” don’t all compete for the same chemicals and nutrients substantially reduces competition for resources within the biofilm.33)
- Disbanding when there are too many freeloaders
A study of a cultured E. coli colony (not necessarily in a biofilm state) found that individual microbes can act altruistically through a form of kin selection.34) Essentially, they sacrifice themselves so that their fellow bacteria have a better chance at survival.
Quorum sensing
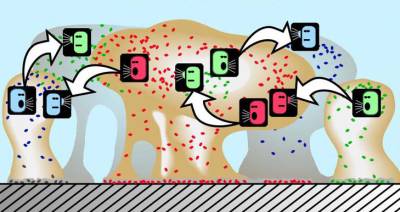
The bacteria that become part of a biofilm engage in quorum sensing, a type of decision-making process in which behavior is coordinated through a “chemical vocabulary.”35) Although the mechanisms behind quorum sensing are not fully understood, the communication process allows, for example, a single-celled bacterium to perceive how many other bacteria are in close proximity. If a bacterium can sense that it is surrounded by a dense population of other pathogens, it is more inclined to join them and contribute to the formation of a biofilm.
Quorum sensing can occur within a single bacterial species as well as between diverse species, and can regulate a host of different processes, essentially serving as a simple communication network. A variety of different molecules can be used as signals.
For example, researchers at the University of Iowa (several of whom are now at the University of Washington) have spent the last decade identifying the molecules that allow the bacterial species P. aeruginosa to form biofilms in the lungs of patients with cystic fibrosis.36)
Singh and his colleagues finally discovered that P. aeruginosa uses one of two particular quorum-sensing molecules to initiate the formation of biofilms. In November 1999, his research team screened the entire bacterial genome, identifying 39 genes that are strongly controlled by the quorum-sensing system.
In a 2000 study published in Nature, Singh and colleagues developed a sensitive test which shows P. aeruginosa from cystic fibrosis lungs produces the telltale, quorum-sensing molecules that are the signals for biofilm formation.37)
Effectiveness of pulsed low dose antibiotics against biofilm formation
Penetration of biofilm
Persisters
Olmesartan against biofilm formation
OlmesartanMedication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor. Also known by the trade name Benicar. 's action as VDR agonistA substance such as olmesartan (Benicar) or 1,25-D which activates the Vitamin D Receptor and transcribes the genes necessary for a proper innate immune response. makes it a critical component of the Marshall Protocol. One of the proteins that the VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response. transcribes most strongly is the antimicrobial peptide, cathelicidin Family of antimicrobial peptides found primarily in immune cells and transcribed by the Vitamin D Receptor.. A 2011 study showed that the cathelicidin LL-37 exhibited effective anti-microbial, anti-attachment as well as anti-biofilm activity of Staphylococcus aureus at concentrations in the low ug/ml range.38)
Images and illustrations of biofilm bacteria
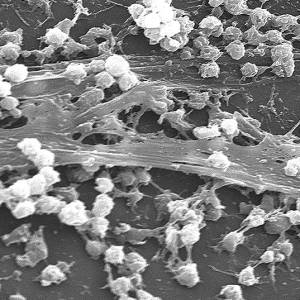
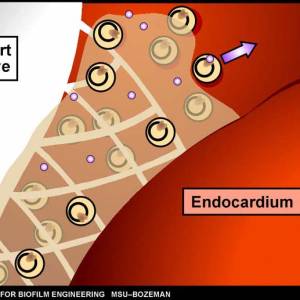
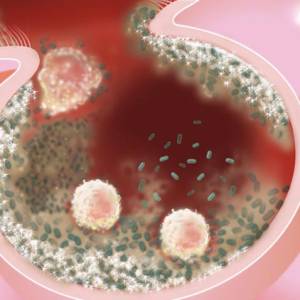
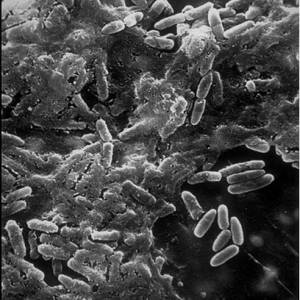

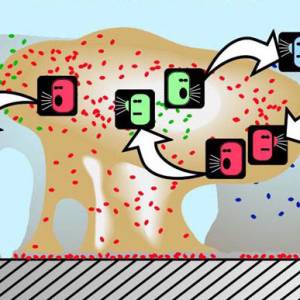
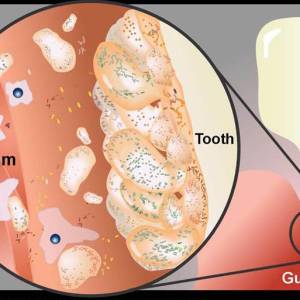
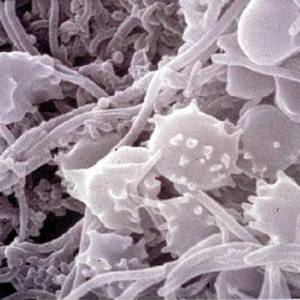
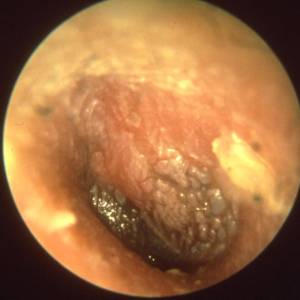
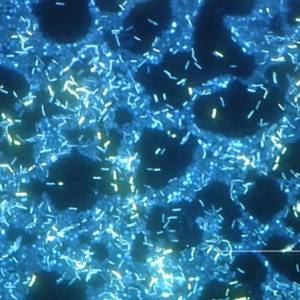
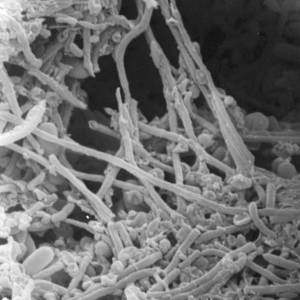
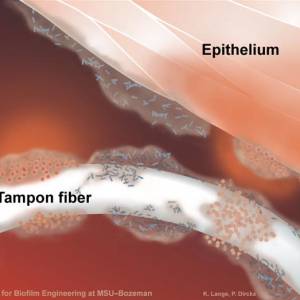
More research
Chronic Middle Ear Infections 39)
Biofilms and chronic infections 40)
From Koch's postulatesCentury-old criteria designed to establish a causal relationship between a causative microbe and a disease. Koch's belief that only one pathogen causes one disease has now been called into question as multiple postulates are increasingly considered out of date. to biofilm theory. The lesson of Bill Costerton. 41)
A specific response to pH by isolates of Haemophilus influenzae 42)
2017 Wound Biofilm: Current Perspectives and Strategies on Biofilm Disruption and Treatments 43)
2017 Proteinase K treatment with antibiotics showed a synergistic effect against S. aureus biofilms. 44)
The role of extracellular DNA in the maintenance of biofilms 45)
Increased intestinal permeability as a direct effect of treatment with a bacteriophage cocktail 46)
2017 Novel insights into the role of bacteriophages as potentially pathogenic for mammals 47)
Read more
[PMID: 19067751] [DOI: 10.1111/j.1574-6976.2008.00150.x]
[PMID: 29235402] [DOI: 10.1080/14787210.2018.1417036]
[PMID: 10334980] [DOI: 10.1126/science.284.5418.1318]
[PMID: 15040259] [DOI: 10.1038/nrmicro821]
[PMID: 11257008] [PMCID: 90417] [DOI: 10.1128/AAC.45.4.999-1007.2001]
[PMID: 16490835] [DOI: 10.1161/CIRCULATIONAHA.105.579979]
[PMID: 16490832] [DOI: 10.1161/CIRCULATIONAHA.105.607358]
[PMID: 16730544] [DOI: 10.1016/j.otohns.2006.03.001]
[PMID: 19418781] [DOI: 10.12968/jowc.2009.18.2.38743]
[PMID: 18086294] [DOI: 10.1111/j.1524-475X.2007.00321.x]
[PMID: 14980298] [DOI: 10.1016/s1526-0542(04)90065-6]
[PMID: 15482158] [DOI: 10.1586/14787210.1.4.609]
[PMID: 18033823] [DOI: 10.1099/jmm.0.47331-0]
[PMID: 16835426] [PMCID: 1885379] [DOI: 10.1001/jama.296.2.202]
[PMID: 14527295] [DOI: 10.1146/annurev.micro.57.030502.090720]
[PMID: 18451039] [DOI: 10.1099/mic.0.2007/014746-0]
[PMID: 19797556] [DOI: 10.14219/jada.archive.2009.0049]
[PMID: 11113379] [DOI: 10.1016/s1286-4579(00)01316-2]
[PMID: 17699815] [DOI: 10.1056/NEJMoa061588]
[PMID: 12843396] [DOI: 10.1126/science.1084550]
[PMID: 17276630] [DOI: 10.1016/j.vetmic.2006.12.029]
[PMID: 21697011] [DOI: 10.1016/j.ijheh.2011.05.009]
[PMID: 11807075] [PMCID: 134825] [DOI: 10.1128/jb.184.4.1140-1154.2002]
[PMID: 16272368] [DOI: 10.1099/mic.0.28295-0]
[PMID: 20059548] [DOI: 10.1111/j.1574-6976.2009.00200.x]
[PMID: 19242455] [DOI: 10.1038/4571083a]
[PMID: 17055982] [DOI: 10.1016/j.cub.2006.08.068]
[PMID: 20811456] [PMCID: 2936489] [DOI: 10.1038/nature09354]
[PMID: 18004304] [DOI: 10.1038/nature06284]
[PMID: 11048725] [DOI: 10.1038/35037627]
[PMID: 21605457] [PMCID: 3397408] [DOI: 10.1186/1471-2180-11-114]
[PMID: 18544729] [DOI: 10.1001/jama.299.22.2682]
[PMID: 23138704] [DOI: 10.5301/ijao.5000169]
[PMID: 24555828] [PMCID: 3938079] [DOI: 10.1186/1471-2180-14-47]
[PMID: 28682297]
[PMID: 29205189] [PMCID: 5735565] [DOI: 10.4103/ijmr.IJMR_410_15]
[PMID: 19064900] [PMCID: 2650517] [DOI: 10.1128/AAC.00471-08]
[PMID: 27340433] [PMCID: 4918031] [DOI: 10.1186/s13099-016-0109-1]
[PMID: 28765534] [PMCID: 5539208] [DOI: 10.1038/s41598-017-07278-6]