Related articles: 5 key elements of MP

Table of Contents
Marshall Protocol
This document is a one-article summary of key issues related to the Marshall Protocol, especially those relevant to physicians. Many of the topics covered here are reviewed in greater depth throughout the Knowledge Base.
Without active participation of patients on MarshallProtocol.com site, Autoimmunity Research Foundation does not support or license the public use of this therapy.
Available translations
Translations of this document (of emerging quality) are available in several languages.
Resources for physicians
We strongly urge physicians and patients to take advantage of the following websites:
- AutoimmunityResearch.org – Visit AutoimmunityResearch.org for Foundation information and links to patient stories.
- MPKB.org – The Marshall Protocol Knowledge Base contains links to peer-reviewed research from the Foundation as well as articles about hundreds of topics written for a variety of audiences.
- MarshallProtocol.com – The MarshallProtocol.com site helps patients and physicians better understand and engage with details about the application of the Marshall Protocol. It contains science-related discussions and patient reports of response to the protocol. The site has open registration for physicians, patients, and those considering the protocol.
- Visual presentations videos, congresses and uploads
- Private Section for Health Professionals forum - Healthcare providers are encouraged to join the conversation in this forum on the MarshallProtocol.com study site, which is open by email request.
While the physician is responsible for patient care, patients can do at least some of the footwork, retrieving information so that the physician can make fully informed decisions.
Background and scientific rationale for the therapy
Related articles: 5 key elements, Science behind Pathogenesis, Science behind Protocol, Grappling with uncertainty
The Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. (MP) is the name given to a therapy devised by Professor Trevor Marshall. Based on the pathogenesis Marshall has proposed for chronic inflammatory disease, the MP is aimed at targeting bacteria, fungi, viruses, and other microbes that appear to interact to cause chronic inflammatory diseases.
Marshall and colleagues have hypothesized that chronic inflammatory diseases, including many autoimmune diseases, are caused by dysbiosis of a metagenomic microbiotaThe community of bacterial pathogens including those in an intracellular and biofilm state which cause chronic disease.: communities of microbial pathogens, many of which persist intracellularly. A recent (2009) peer-reviewed paper describes this Pathogenesis in more detail.1)
Supported by Autoimmunity Research FoundationNon-profit foundation dedicated to exploring a pathogenesis and therapy for chronic disease., the MP has been available since 2002 and has been used in a wide range of chronic inflammatory illnesses.
A significant number of patients diagnosed with sarcoidosis, post-treatment chronic Lyme syndrome, chronic fatigue syndrome, uveitis, Hashimoto’s thyroiditis, rheumatoid arthritis, fibromyalgia, diabetes type II, psoriasis, lupus (SLE), multiple sclerosis, and a number of other diagnoses are showing a promising response from being treated with the Protocol.
In determining whether a patient can be successfully treated with the MP, a specific chronic disease diagnosis is not as important as the clinical assessment by an interested health care provider, the results of a therapeutic probe, and outcome of the vitamin D metabolites blood test.2)
According to the Marshall PathogenesisA description for how chronic inflammatory diseases originate and develop., chronic inflammatory disease is characterized by dysregulation of the nuclear receptorIntracellular receptor proteins that bind to hydrophobic signal molecules (such as steroid and thyroid hormones) or intracellular metabolites and are thus activated to bind to specific DNA sequences which affects transcription. pathways which control the innate immune responseThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease.. For example, the Vitamin D nuclear receptorA nuclear receptor located throughout the body that plays a key role in the innate immune response. (VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response.) expresses many of the body's antimicrobial peptidesBody’s naturally produced broad-spectrum antibacterials which target pathogens. (along with TLR2A receptor which is expressed on the surface of certain cells and recognizes native or foreign substances and passes on appropriate signals to the cell and/or the nervous system.). In addition to down-regulation of expression of the VDR itself by many common bacteria and viruses, antagonistic microbial metabolites incrementally block ligands from activating it. Ingested vitamin D slows activity of the receptor in this same manner, preventing the body from killing the pathogens at the heart of the disease state. That is why avoidance of ingested vitamin D (in food and supplements) is essential for the innate immune system to recover. 3) 4)
The MP uses multiple daily dosing of olmesartan medoxomil (Benicar, Olmecip, Olmetec) to re-activate the Vitamin D Nuclear Receptor, dislodging bacterial ligands in the process. This drug was developed as an Angiotensin II Receptor Blocker (ARBA drug which is an angiotensin receptor blocker. One of the ARBs is olmesartan (Benicar). Not all ARBs activate the Vitamin D Receptor.) but it has multiple actions in the human body when dosed as defined by Marshall. In addition to immunostimulation via the VDR, OlmesartanMedication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor. Also known by the trade name Benicar. also reduces inflammatory cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. production by inhibiting the NF kappa-B transcription pathway. This inhibits, among other things, the release of TNF-alphaA cytokine critical for effective immune surveillance and is required for proper proliferation and function of immune cells., helping to protect the organs from effects of excessive inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue..
Additionally, several pulsed, low-dose, bacteriostatic oral antibiotics may optionally be used, in a minority of patients it may reduce IP, but is more likely to provoke it when after some months, it has declined to insignificance. Four bacteriostatic antibiotics: minocycline, clindamycin, sulfamethoxazole-trimethoprim (Bactrim DS), and demeclocycline (Declomycin) have been found useful for these outcomes. Minocycline directly acts in an immunosuppressive manner on the PXR nuclear receptor, and this biochemical action may be useful in pulsing immunopathologyA temporary increase in disease symptoms experienced by Marshall Protocol patients that results from the release of cytokines and endotoxins as disease-causing bacteria are killed. to (for example) a 48 hour cycle. Bactrim, however, may be better avoided by anyone with a tendency to high potassium MedPage which is not under control by sodium bicarbonate (see baking soda)
N.B. azithromycin is no longer recommended for use while on the MP.
Seriously ill patients may develop photosensitivityAbnormal sensitivity to sunlight and bright lights. Also referred to as "sun flare" or "light flare." during the healing process, so avoidance of direct and indirect sunlight may be necessary. Patients may need to protect their eyes from bright lights to prevent further retinal damage and reduce neurological symptoms due to inter alia, the effect of ocular 1,25-DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol. production on the brain.
Patients may also develop sensitivity to skin exposure to sunlight, and/or find that they need to avoid skin exposure to sunlight in order to maintain the naturally low blood levels of vitamin D required by the Protocol. However, some patients do not experience significant photosensitivity during recovery. Those who do often find it more manageable several years into the therapy.
Immunopathology
Main article: Science behind immunopathology
Related articles: Managing immunopathology, Immunopathology
When patients on the MP kill bacterial pathogens they experience a reaction called immunopathology. Immunopathology is an increase in one's present symptoms of Th1 inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue., or a return of previous Th1 inflammatory symptoms, that is caused largely by cytokinesAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. generated by the immune response and endotoxins released from dying bacteria. Occasionally, immunopathology will result in a new symptom or abnormal laboratory value (e.g., elevated creatinine, elevated liver enzymes, low white blood count, etc.). The occurrence of subclinical bacterial inflammation is due to olmesartan's activation of the immune system. Immunopathology appears to be a necessary part of recovery. The amount of immunopathology a patient experiences on the Marshall Protocol (MP) tends to correlate with disease severity and bacterial load. Patients who are less sick will have comparatively less-strong immunopathology.
Immunopathology is sometimes used synonymously with the “Jarisch-Herxheimer reaction” or “herx.”
Many MP patients who have experienced prolonged periods of immunopathology have reached stages of significant improvement or remission. This supports the conclusion that immunopathology is a necessary result of chronic bacterial death, and a precursor to disease reversal. The MP is not unique in this regard. A number of other diseases and/or therapies generate immunopathological or immunopathological-like reactions.5) 6) 7)
Lab work and patient reports can be used to track clinical signs of immunopathology.
Patient eligibility and prerequisites
The Marshall Protocol has been used in a variety of chronic inflammatory diseases. The gold standard for evaluating whether the MP is warranted is the therapeutic probeA brief trial of the Marshall Protocol to see if it will generate an immunopathological response. The "gold standard" for testing whether a patient is a good candidate for the MP., a brief trial of the Marshall Protocol to see if olmesartan medoxomil and pulsed low-dose minocycline will generate an immunopathological response. The results from a vitamin D metabolites test, while less definitive, may also suggest the presence of treatable condition. For an automated interpretation of the vitamin D metabolites, consult the Vitamin D Metabolite Calculator.
Other patient groups:
- pregnant/lactating women – Both olmesartan and minocycline, the two mainstay medications of the MP, are contraindicated during pregnancy and while breastfeeding. Women of childbearing age on the MP should take adequate contraceptive precautions.
- children – Children with certain diseases and conditions have been treated with the MP. There are more than a dozen in the study cohort who are doing well. The FDA recently approved the use of olmesartan for hypertensive children ages 6-16.
Before commencing therapy, physicians and patients should familiarize themselves with the pre-MP checklist, which reviews the medications, eye protection, and possible lifestyle modifications necessary for treatment success and safety.
Safety considerations
Main article: Safety warnings for the Marshall Protocol
- Some patients' immunopathology may be difficult to control - Immunopathology in patients with severe forms of disease may be difficult to control. Physicians should help their patients learn the strategies for managing immunopathology. Knowing how to manage the MP independently on a day-to-day basis will serve patients well should an urgent situation occur. Patients should be urged to seek support from the Foundation's websites.
- Patients on the MP should not take nimesulide (Aulin / Mesulid / Nimed). It could cause bleeding. One death has been reported during its use.
- Rivaroxaban (Xarelto) increases the risk of bleeding and can cause serious or fatal bleeding. A specific antidote for rivaroxaban is not available.
- Do not give Telmisartan, it has an over strong action in suppressing the patient's ability to fight infection.
- Azithromycin is no longer regarded as safe, even in the small quantities formerly recommended for optional use while on MP
- For sicker patients, immunopathology can be physically and mentally challenging – Support from a knowledgeable doctor and family/friends becomes of paramount importance. Unless the patient has a good insight into IP and other inconveniences of the treatment, the patient should not start treatment.
Non-MP treatments
Main article: Non-MP medications
Related articles: Antibioitics under special circumstances
While there are notable exceptions, the Marshall Protocol (MP) should not be combined with any other protocols, treatments or supplements, especially those which are immunosuppressive or immunomodulatory. Using other treatments while on the MP can impede progress on the MP – or be dangerous to MP patients.
For intolerable symptoms, certain palliative medications such as sleep medication, pain medication, and antidepressants are acceptable for short term use. It is generally recommend that MP patients use the lowest dose of medication that is effective.
The following is a summary of common medications that have the potential to interfere with the MP. A more complete list of medications is available in the Non-MP treatments article.
- antibacterials – Sulfusalazene, Plaquenil and Methotrexate are antimetabolite antibiotics with actions similar to Bactrim and may produce unwanted or uncontrolled immunopathology. They must be discontinued before starting the MP.
- antibiotics for acute infections – Olmesartan greatly potentiates the action of many antibiotics. Consequently, a severe or life-threatening immunopathological reaction may result if non-MP antibiotics are prescribed for an acute infection. Olmesartan should not be withdrawn during these periods as it protects vital organs from damage by cytokinesAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system.. If a non-MP antibiotic is temporarily needed for an acute infection, please follow the instructions in the Notice for emergency medical personnel.
- anticoagulants – Olmesartan may profoundly affect the anticoagulant dosing requirement. It is absolutely essential to closely monitor any patient on anticoagulant therapy.
- antifungal agents – Medicines such as Diflucan and Lamisil are known to interfere with vitamin D metabolism and are generally contraindicated.
- antiviral agents – Antivirals may have profound effects on the immune system as well as a number of serious adverse effects. Routine use is generally contraindicated for MP patients.
- bisphosphonates – May cause calcium deposition into the soft tissues, reduced organ function and possible osteonecrosis of the jaw.
- calcium supplements – Should be avoided in the presence of hypercalciuria or hypercalcemia. Those without these conditions should generally consume 1000-1500 mg calcium daily, preferably from whole foods, but if this is not possible, calcium supplements without vitamin D are acceptable.
- corticosteroids – Therapy will not be effective while corticosteroidsA first-line treatment for a number of diseases. Corticosteroids work by slowing the innate immune response. This provides some patients with temporary symptom palliation but exacerbates the disease over the long-term by allowing chronic pathogens to proliferate. are suppressing the immune system. Begin or continue the gradual weaning process with the assistance of palliation from olmesartan. ACTH and cortisol production may be monitored to assess adrenal function.
- diuretics – Some diuretics including the thiazides are contraindicated for MP patients. If a diuretic is necessary, Lasix is preferred.
- folic acid (prescription or over the counter) – Folic acid makes it easier for occult bacteria to replicate and create new DNA. Consuming a balanced diet low in supplemental folic acid should provide adequate folic acid.
- hormonal replacement therapy (e.g. progesterone, estrogen and testosterone) – Because hormonal supplementation can interfere with the activity of many of the nuclear receptorsIntracellular receptor proteins that bind to hydrophobic signal molecules (such as steroid and thyroid hormones) or intracellular metabolites and are thus activated to bind to specific DNA sequences which affect transcription., hormonal supplementation is contraindicated. However, under certain circumstances, patients may continue hormone replacement therapy, until they are able to wean from it. See article on hormonal replacement therapy.
- interferon therapy – Interferon therapy is immunosuppressive, reducing in number both cytokines and immune cells.
- intravenous immunoglobulin (IVIG) – IVIG is derived from the blood of a pool of more than a hundred individuals, who could together harbor a large number of pathogens. There is a significant likelihood that pathogens from the blood of these people might spread to the recipient.
- nimesulide (Aulin / Mesulid / Nimed) may cause internal bleeding.
- rivaroxaban (Xarelto) increases the risk of bleeding and can cause serious or fatal bleeding. A specific antidote for rivaroxaban is not available.
- statins – Statins have a range of documented negative side effects and have been shown to exacerbate certain kinds of chronic disease. Some compete for the VDR binding pocket. They are contraindicated.
- thyroid supplements – Need for these supplements may decline within a day or two of being on olmesartan alone. Monitor thyroid function closely and adjust the level of thyroid supplementation downward as needed.
- TNF-alpha blockers – Tumor necrosis factor-alpha or TNF-alpha is a cytokine critical for effective immune surveillance. Anti-TNF drugs, also known as TNF-alpha blockers, are drugs which interfere with the body's production of TNF-alpha. While olmesartan also reduces levels of TNF-alphaA cytokine critical for effective immune surveillance and is required for proper proliferation and function of immune cells., it does so to a lesser extent and in a manner less detrimental to immune function. Olmesartan blocks the initial generation of the substance rather than disabling free-floating TNF-alpha after the cytokine has been produced.
Food and drink
Main article: Food and drink
Patients on the MP must avoid all food and drink that contains supplemental vitamin D or high levels of naturally-occurring vitamin D. MP patients must avoid foods and drinks high in chlorogenic acidAn antioxidant and phenolic compound which in ways that are not yet fully clear can modulate and/or suppress the immune response – particularly coffee, concentrated juices, and supplements and multivitamins containing added folic acid. A low-carbohydrate, insulin-resistant diet is recommended for MP patients but is not required. Specific nutritional imbalances should in some cases be corrected, but this requires proper understanding of both the MP and the nutritional needs of the body by a health professional.
Photosensitivity
Main article: Photosensitivity
Abnormal sensitivity to sunlight and bright lights is known as photosensitivity and sometimes referred to as “sun flare” or photophobia. In the context of the MP, the ultimate cause of photosensitivity is the Th1 inflammatory disease process – not the treatment itself. Exposure to natural or bright artificial light in a photosensitive person can lead to flares of internal disease activity, including exacerbation of any inflammatory disease symptoms. Neurological phenomena also occur since the amygdala has nerves connecting it to a number of important brain centers, including the neocortex and visual cortex.
Photosensitivity can occur either when the skin is exposed to bright natural light or the eyes are exposed to either natural or artificial light. Photosensitivity symptoms can occur immediately after exposure or begin 1 to 3 days later, sometimes persisting 5 days or more.
Individuals who are photosensitive prior to the MP will likely become more photosensitive on the MP. Individuals who have no signs of photosensitivity may or may not become photosensitive on the MP. Individuals with limited inflammatory symptoms (suggesting early disease) are the most likely to be able to tolerate more light exposure while on the MP. There is no certain way to tell in advance precisely how photosensitive an individual will be while on the MP. Only after an individual has begun treatment can photosensitivity be assessed.
For many members but not all it is prudent to block sunlight from living space, work space and practice limited sunlight exposure and cover up skin with thick dark layers when going out as well as protect the eyes with the proper NOIRSpecial sunglasses worn by Marshall Protocol patients to block light. glasses.
When in doubt, patients should assume their intolerable symptoms are due to light exposure and reduce sunlight exposure and protect eyes with the proper NOIR sunglassesSpecial sunglasses worn by Marshall Protocol patients to block light..
Patients on the MP often benefit from wearing glasses that block a broader spectrum of light and in many cases must cover their skin when in the sun. Further guidelines are available at the Knowledge Base articles on Eye protection and Skin protection.
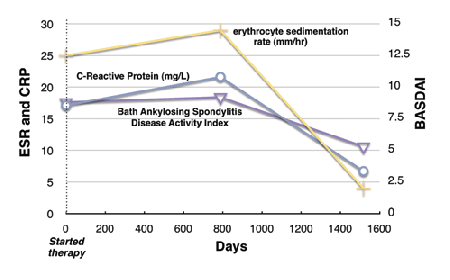
Laboratory tests
Main article: Laboratory tests
Most patients on the MP experience temporary but well-defined increases in various markers of disease state and inflammation, consistent with an immunopathological response. It is helpful, but not necessary, to measure % lymphocytes, C-Reactive Protein, alkaline phosphatase, triglycerides, relevant “autoantibodies”, and serum ACE, to track systemic inflammation. Doctors may want to assess kidney function by testing creatinine or BUN and measure other indicators specific to each patient for a baseline and retest as appropriate. Some lab work – commonly HGB, HCT, eGFR, creatinine and BUN – may become temporarily abnormal, due to immunopathology reactions, until the inflammation resolves.
For example, a higher than usual BUN and creatinine is not an indication that olmesartan should be discontinued but a sign that immunopathology may be occurring in the kidneys or other nearby organs. In most cases where physicians have allowed such levels to remain temporarily out of range, BUN and creatinine have returned to range as microbial die-off in the kidneys subsides. We are not aware of any reports of MP patients needing dialysis, provided they remained on olmesartan.
If these markers indicate dysfunction sustained for more than several months, we advising using one or more methods to lower immunopathology levels.
See also Kidney information
Vitamin D metabolites
Related article: Vitamin D metabolite test instructions
There are two main vitamin D metabolites:
- 1,25-dihydroxyvitamin DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol. (1,25-D) – The presence of high levels of this active vitamin D hormone in patients suggests an ongoing chronic inflammatory disease process.9) However, the absence of elevated levels does not mean a patient cannot benefit from treatment particularly since non-MP therapies such as TNF-alpha blockers and other unknown drugs and interventions can alter 1,25-D levels. Whenever possible, use the lab Quest Diagnostics. Quest has recently ceased to routinely freeze the 1,25-D samples, as we find necessary. However, the 1,25-D sample will not be frozen unless the patient specifically asks.
- 25-hydroxyvitamin D (25-D) – Unlike 1,25-D, serum samples of 25-D need not be frozen. 25-D is a secosteroid with possible immunosuppressive effects.10) Thus, patients on the MP restrict consumption of vitamin D in order to reduce their 25-D at or below 12 ng/ml. Levels of 25-D should be tested every 6-12 months. Be aware that as 25-D levels fall, immunopathology may increase, sometimes dramatically.
If the vitamin D metabolite tests do not indicate Th1 inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue. but clinical observation suggests otherwise, a short course of the MP (1 to 2 months) should be used as a therapeutic probe. A longer time period may be needed if 25-D levels remain high, as a therapeutic probe is often not effective unless 25-D levels fall below 25 ng/ml.
Consult the Vitamin D Metabolite Calculator for suggestions on interpreting this lab data.
Measures of blood pressure
Main article: Low blood pressure and dizziness
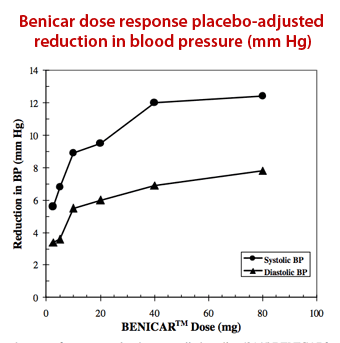
The primary indication for olmesartan (Benicar) is as a mild hypotensive drug. As one can see from the FDA label for Benicar (right), the dose response curve for Benicar is asymptotic, with higher dosages of the drug having incrementally smaller decreases in blood pressure. For example, the difference between 40mg and 80mg of olmesartan results in a decrease of no more than 1mm Hg.
A decline in systolic pressure greater than 15mm Hg of mercury cannot solely be due to olmesartan’s hypotensive action. Instead, the drop is also likely due to the disease processes itself.
For example, the widespread destruction of bacteria and human cells infected by bacteria can lower blood pressure. Although this isn’t true of all bacterial forms, when some forms of bacteria are destroyed, they release endotoxins,11) the bioavailability of which can lead to a steep decline in blood pressure.12)
If a patient suffers low blood pressure before the MP, low blood pressure will return as a symptom of immunopathology while on the MP. In most cases, we find as bacterial die-off subsides, blood pressure levels begin to return to a normal range even as patients continue to take the same dose of olmesartan.
Thus, medications that raise blood pressure, such as fludrocortisone and dopamine, are contraindicated, both because they would do nothing to slow bacterial die-off and because they may have deleterious effects on immune function.
Olmesartan (Benicar)
Related articles: Science behind olmesartan (Benicar), Benefits of olmesartan for patients with kidney disease, Olmesartan safety
For the purposes of the MP, olmesartan has two primary actions: it reduces inflammation by blocking the Nuclear Factor-kappaB cytokine pathway and it is an agonist of the Vitamin D Receptor (VDR). As a VDR agonistA substance such as olmesartan (Benicar) or 1,25-D which activates the Vitamin D Receptor and transcribes the genes necessary for a proper innate immune response., olmesartan activates the innate immune response. Research supports the safety of the doses used by MP patients. Olmesartan has minimal interactions with other drugs and is one of the safest drugs on the market.
The half-life of olmesartan is reported to be 13 hours. This would imply that the drug would remain active during that period of time, however, we have found that in sick patients, olmesartan is most effective when administered every 4-6 hours, with a maximum of every 8 hours. This may be due to the fact that some intracellular infections (notably Shigella), upregulate activity of the caspases, which are proteases that cleave the VDR.13) When the VDR is broken apart by the caspases, it is highly likely that any ligands bound to it (such as olmesartan) would stay bound to the fragments of the protein. Therefore, a VDR agonist would be effective over shorter periods of time in patients with infected cells.
The U.S. Food and Drug Administration has set no safe limit for olmesartan medoxomil (Benicar), as no dose-related adverse events have been identified to this point. FDA post-marketing-experience has shown that Olmesartan has one of the safest profiles of any drug on the market. Note that this does not apply to the combination drugs, such as Benicar HCT, which contains a thiazide and is harmful, and should never be used with an MP dosing schedule.
The label for olmesartan medoxomil states that the drug is well-tolerated. Adverse events were similar to those experienced by the placebo group. Adverse events were generally “mild, transient and not related to dose.” The frequency of adverse events also had no relationship to the dose of olmesartan.
A 2001 study published in the Journal of of Pharmacology found olmesartan to be safe and well tolerated at dosages of up to 160 mg/day.14)
In placebo-controlled trials, the only side effect that occurred in more than 1 percent of olmesartan-treated patients vs. placebo-treated patients was dizziness (3 percent vs. 1 percent).15)
The relevant Knowledge Base article reviews the safety profile of olmesartan/Benicar in greater detail.
Antibiotics
The Marshall Protocol has historically emphasized a role for antibiotics. However, as our understanding of the recovery process has progressed, this emphasis has been greatly minimized. This change is gradually being reflected in the guidance available here. In the event of any uncertainty, patients or their physicians should seek guidance on the Protocol forums.
Starting a patient on the Marshall Protocol
- Test vitamin D metabolites – Follow the vitamin D metabolites testing instructions. Remind the drawing lab that the 1,25-D sample must be clotted no more than 30 minutes before centrifuge and the resulting serum must be frozen for shipment. Consult the Vitamin D Metabolite Calculator for suggestions on interpreting this lab data.
- Restrict dietary vitamin D intake – Patient must restrict all supplements and foods high in Vitamin D. It is recommended that, over the course of treatment, the patient reduce 25-D to the therapeutic target of approximately less than 12 ng/ml. Retest 25-D periodically to make sure 25-D is dropping toward the lower end of the therapeutic range. Many MP patients have kept their 25-D below 5 ng/ml for many years, without any adverse effect.
- For patients taking corticosteroids, begin olmesartan – Corticosteroids are contraindicated for MP patients. Before weaning them, patients should first begin olmesartan (see below), which can greatly relieve withdrawal symptoms and help ensure weaning success. It is recommended that olmesartan be started a week or two before beginning to wean. See the weaning guidelines for detailed instructions.
- Withdraw or begin to wean contraindicated therapies
- If necessary, avoid light – If necessary to avoid the symptoms of photosensitivityAbnormal sensitivity to sunlight and bright lights. Also referred to as "sun flare" or "light flare.", patients should avoid outdoor light and bright indoor lights by staying indoors as much as possible, using heavy curtains or window shades, and covering up well whenever venturing outside during daylight hours. Patients may also need to protect their eyes from both outdoor and indoor light.
- Begin olmesartan – Commence therapy by prescribing 40mg pure olmesartan medoxomil every six hours (e.g.: 6am, noon, 6pm, midnight) to interrupt the inflammatory cycle and reduce the severity of potential immunopathology. Our observations suggest that olmesartan medoxomil is the only angiotensin receptor blocker (ARBA drug which is an angiotensin receptor blocker. One of the ARBs is olmesartan (Benicar). Not all ARBs activate the Vitamin D Receptor.) that activates the patient’s innate immune system. “No substitutions” should be written on the prescription. Avoid any combination formulation such as Benicar hydrochlorothiazide (Benicar HCT). Because patients often begin to feel worse when decreasing light and/or vitamin D, olmesartan should be prescribed concurrently with the previous steps, so that it can palliate any resulting immunopathology while the 25-D levels are decreasing. Patients should keep several weeks’ supply of olmesartan in reserve to use in case it is needed to treat intolerable immunopathologyAn unbearable or unsafe severity of bacterial die-off reaction..
- Wait for patient to stabilize on olmesartan – It usually takes a month or two to stabilize symptoms on olmesartan alone. Some patients may need more time. Depending on the patient's bacterial load and a host of other factors, some patients initially feel better on olmesartan, some worse, and some don't experience any change. All three reactions are normal. A partial list of typical immunopathology symptoms includes: depression, irritability, mania, paranoia, fatigue, muscle weakness, rash, headache, photosensitivity, pain anywhere, numbness, nausea, diarrhea, constipation, ringing in the ears, toothache, sinus congestion, nasal stuffiness, fever/chills, flu-like body ache, cough, sleep disturbances and “brain fogThe loss of intellectual functions such as reasoning; memory loss; and other neurological abilities that is severe enough to interfere with daily functioning..”
- There may be benefit from olmesartan every four hours - the immunostimulative and palliative effects of olmesartan are believed to be maximal at four-hourly dosing. Once the patient has stabilized, the frequency should be gradually increased to every four hours, over several weeks, or in accordance with the patient's ability to tolerate any increases in immunopathology that may result. (Some patients will actually feel better at the higher frequency.)
Managing immunopathology
Main article: Managing immunopathology
Related articles: Immunopathology, Science behind immunopathology
Patients' goal during the MP should be to maintain tolerable immunopathologyA state in which a patient has maintained an acceptable intensity of bacterial die-off reaction. The primary goal of the Marshall Protocol. as they get well. In cases where IP is becoming intolerable, certain strategies are available including:
- Adjust olmesartan (Benicar) - Depending on which of olmesartan's two main properties a patient experiences most strongly during a particular period of treatment, increasing or decreasing the dose may help manage immunopathology. For some, taking olmesartan sublingually (under the tongue) can provide immediate relief.
- Minocycline as a palliative – Minocycline taken more frequently such as every 12-24 hours, it may help reduce symptoms of immunopathology.
- Take palliative medications – A range of symptom-specific palliative medications can be relied upon in the case of intolerable immunopathology - enquire in the forums at MarshallProtocol.com
- Light restriction. Sunlight to the skin and eyes can exacerbate symptoms and make symptoms intolerable. This is individual. However, if experiencing intolerable symptoms one will want to reassess their response to every type of light exposure. Indoor lighting above 30 lux or any type of fluorescent lighting has been enough provoke intolerable symptoms for some patients, even when wearing NoIR sunglasses.
Note that three forms of IP are particularly life-threatening and should be handled with an abundance of caution: cardiac immunopathology, neurological immunopathology, and respiratory immunopathology. Patients who are concerned about any of these or other symptoms should not hesitate to call their physician.
In case of emergency
Main article: Notice for emergency medical personnel
ARF has prepared a Notice for emergency medical personnel treating a Marshall Protocol patient. Important points from that document include the following:
- Do not withdraw olmesartan – In a critical care situation, it is essential to continue oral olmesartan, even in the presence of hypotension, as abrupt withdrawal can be life-threatening. Along with routine lifesaving procedures, it is essential to continue oral olmesartan 40mg dosing every four hours, with 20mg SL p.r.n., until symptoms subside - even if an NG tube is necessary. If B/P is extremely low (mean arterial pressure <55), continue olmesartan as above and increase fluid volume with 0.9 NS or packed red cells.
- antibiotics – We strongly recommend patients not be treated with MP antibiotics for an acute infection. Unless patients have reached a late stage of the treatment these antibiotics may greatly increase immunopathology as they leave a patient's system. Cephalosporins, Claforin, and the macrolide Biaxin are usually tolerated. Fluoroquinolone antibiotics may be tolerated although instances of tendon damage have been reported; the patient should be advised of the FDA black-box warnings.
- corticosteroids – Do not give corticosteroids in any form or by any route (injected, inhaled, oral or IV) as they will lead to metabolic instability.
- Do not give nimesulide (Aulin / Mesulid / Nimed). It could cause bleeding.
- Rivaroxaban (Xarelto) increases the risk of bleeding and can cause serious or fatal bleeding. A specific antidote for rivaroxaban is not available.
- epinephrine or norepinephrine – Adverse reactions may occur if epinephrine or norepinephrine is used to raise B/P or treat anaphylaxis. Use epinephrine and norepinephrine only for cardiac arrest. Local anesthetics containing epinephrine may cause adverse events (tachycardia, psychosis), and the epinephrine may hinder anesthesia.
For the details of these recommendations, please consult the Notice for emergency medical personnel.
In an emergency, physicians may call Trevor Marshall at 1-805-492-3693.
Length of the Protocol
Main article: Length of the Protocol
Related article: Spontaneous remission is a myth
The exact duration of the Marshall Protocol (MP) depends on any number of factors, including degree of illness, amount of fibrosis, ability of the kidneys to process and expel breakdown material, subclinical inflammation, exposure to unavoidable immune suppressants, and personal preference to remain on Olmesartan.
While someone who is very ill might expect the MP to take five or more years, there is no way to know for sure how long the treatment will take. Due to the nature of immunopathology, feelings of well-being and blood markers of disease tend to be variable in the short-term and improve over the long-term. Also owing to the nature of infection, different symptoms will improve at different rates.
So long as one is responding to olmesartan or olmesartan plus antibiotics with symptoms that wax and wane, there are still bacteria to be killed.
Note that there is no requirement for patients to use antibiotics in order to complete the Protocol. In many cases, patients can make considerable progress on olmesartan (Benicar) alone as the drug increases expression of the body's own antimicrobial peptidesBody’s naturally produced broad-spectrum antibacterials which target pathogens..
If choosing to use antibiotics there is no need to reach the maximum dosages for all antibiotics or do all antibiotic combinations. However, it is considered a good indication of patients' return to health if they no longer experience immunopathology from any antibiotic combination.
Endpoints of the Protocol
Related article: Aiming at health
To a large extent, patients who have completed the Marshall Protocol can return to a normal life with the following modifications:
- consumption of vitamin D – former MP patients are free to enjoy foods such as fish that naturally contain vitamin D. Even so, patients are warned not to consume any food products that are fortified with extra vitamin D.
- light – Although suntanning is not an option, veterans of the MP may choose to expose their eyes and skin to increasing amounts of light. A word of caution: some patients in later stages of treatment may experience a Stage Five reaction when exposed to too much light. To limit the possibility of a severe immunopathological reactionA temporary increase in disease symptoms experiences by Marshall Protocol patients that results from the release of cytokines and endotoxins as disease-causing bacteria are killed., always increase light exposure (and exercise) gradually. Also, be aware that sometimes an increase in symptoms from light may begin one or two days after exposure and last for several days or even longer.
- radio frequency radiating technology - prudent use within household, setting cell phones to Airplane mode when not in use, switching commputer modem off at bed time, measuring and avoiding exposure in other places whenever possible.
- antibiotics – The recovering immune system is self-sustaining and will eradicate bacteria when only Olmesartan is taken. However, there is no harm in using a few weeks' worth of MP antibiotics as an annual checkup - to see if they can generate immunopathology.
- olmesartan (Benicar) – In later stages of the MP, the Vitamin D Receptor, which controls key components of innate immunityThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease., is properly working again and is activated even in the absence of olmesartan. However, olmesartan is still essential to palliate symptoms and protect organs from systemic immunopathology.
- laboratory tests – Various tests are expected to come in range:
- return of ACE, CRP, triglycerides, ALP to low end of normal
- increase in % lymphocytes, back into the normal range
- signs of inflammation resolution on CT and MRI imaging
Stopping the Protocol
Related article: Taking a break from the Marshall Protocol
Stopping olmesartan
Olmesartan will need to be weaned very gradually. Note that the immune response may remain activated for a period of time even after discontinuing olmesartan. Patients who stop olmesartan are terminating their recovery.
Data
Success rates for various Dx, compiled in 2013 (extract below)
Recovery figures
TOTALS: 864 members;573 report success; 119 report no success; and for 172 results are not clear.
SUCCESS RATES: Over all success rate 66.32% Over all unsuccessful 13.77% Over all unsure 19.91%
Sarcoidosis success 75.8%
Chronic Fatigue Syndrome success 60.1%
Lyme disease success 66.9%
Rheumatoid arthritis success 69.2%
Fibromyalgia success 64.2%
All Other Th1 diseaseAny of the chronic inflammatory diseases caused by bacterial pathogens. success 59.8%
[PMID: 19393196] [DOI: 10.1016/j.autrev.2009.02.016]
[PMID: 19758177] [DOI: 10.1111/j.1749-6632.2009.04875.x]
[PMID: 19393200] [DOI: 10.1016/j.autrev.2009.02.011]
[PMID: 11997718] [DOI: 10.1097/00005792-200205000-00005]
[PMID: 18600241] [DOI: 10.1038/eye.2008.204]
[PMID: 12604286] [DOI: 10.1054/jocn.2002.1129]
[PMID: 17557889] [PMCID: 1955167] [DOI: 10.1136/ard.2007.069831]
[PMID: 7619330] [DOI: 10.2165/00002018-199512030-00004]
[PMID: 8479465] [DOI: 10.1056/NEJM199305203282005]
[PMID: 17696608] [PMCID: 1941748] [DOI: 10.1371/journal.ppat.0030111]
[PMID: 11361048] [DOI: 10.1177/00912700122010393]
[PMID: 15291377] [DOI: 10.1016/s0149-2918(04)90143-9]


