Related articles: Metabolism of vitamin D and the Vitamin D Receptor , Aiming at health

Table of Contents
Innate immune response and Th1 inflammation
Introduction
The innate immune response is the body's first line of defense against and a non-specific way for responding to bacterial pathogens.1) Located in the nucleus of a variety of cells, the Vitamin D nuclear receptor (VDR) plays a crucial, often under-appreciated, role in the innate immune response. The vitamin D receptor and T cell function
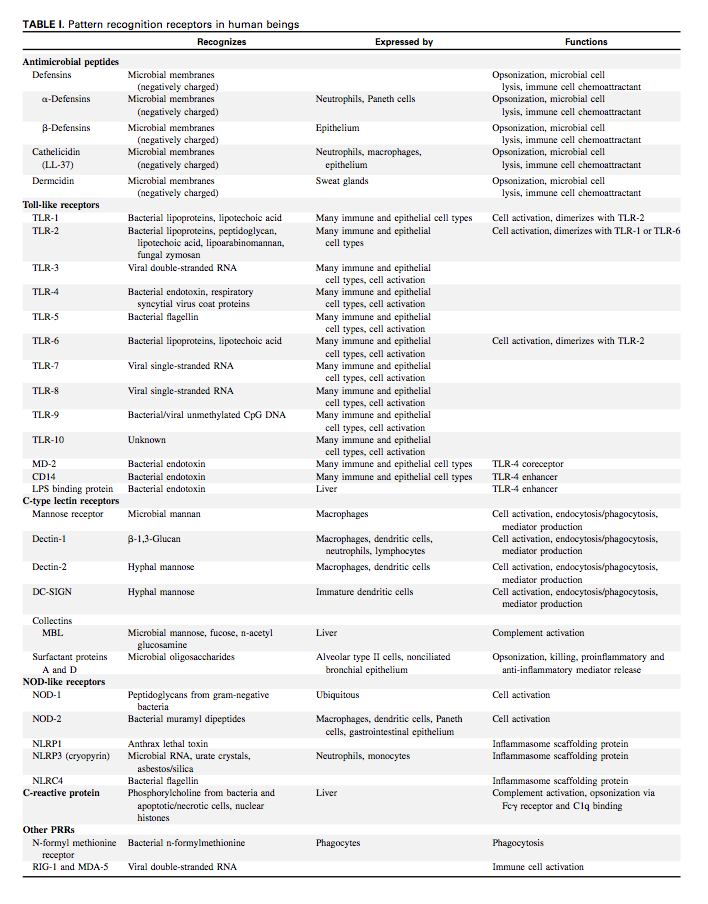
When functioning properly, the VDR transcribes between hundreds2) and thousands of genes3) including those for the proteins known as the antimicrobial peptides. Antimicrobial peptides are “the body's natural antibiotics,” crucial for both prevention and clearance of infection.4) The VDR also expresses the TLR2 receptor, which is expressed on the surface of certain cells and recognizes foreign substances.
The body controls activity of the VDR through regulation of the vitamin D metabolites. 25-hydroxyvitamin D (25-D) antagonizes or inactivates the Receptor while 1,25-dihydroxyvitamin D (1,25-D) agonizes or activates the Receptor.
Greater than 36 types of tissue have been identified as having a Vitamin D Receptor.5)
Another component of the innate immune response is the release of inflammatory cytokines. The result is what medicine calls inflammation, which generally leads to an increase in symptoms.
Adenosine: an endogenous regulator of innate immunity. 6)
Before the Human MicrobiomeThe bacterial community in the human body. Many species in the microbiota contribute to the development of chronic disease. Project, scientists couldn't link bacteria to inflammatory diseases. But with the advent of DNA sequencing technology, scientists have detected many of the bacteria capable of generating an inflammatory response. All diseases of unknown etiology are inflammatory diseases.
Critical illness is a complex life-threatening disease characterized by profound endocrine and metabolic alterations and by a dysregulated immune response, together contributing to the susceptibility for nosocomial infections and sepsis
Hyperglycaemia, as part of the endocrine-metabolic responses to stress, is present in virtually all critically ill patients and is associated with poor outcome.
Not using parenteral nutrition during the first week in intensive care units, and so accepting a large macronutrient deficit, also resulted in fewer secondary infections, less weakness and accelerated recovery.
Research
Those with a high intellectual capacity (hyper brain) possess overexcitabilities in various domains that may predispose them to certain psychological disorders as well as physiological conditions involving elevated sensory, and altered immune and inflammatory responses (hyper body). Science Direct
Modern vaccinations, fear of germs and obsession with hygiene are depriving the immune system of the information input upon which it is dependent. ….and may lead to increased incidences of allergies and autoimmune diseases. 7)
Country kids had more, and more-diverse, bacteria on their skin, with a particularly high abundance of Acinetobacter—a genus of microbes in the Proteobacteria phylum that are commonly found on plants. The researchers further found that children with more Acinetobacter on their skin had more leukocytes in their bloodstream and that these cells were much more capable of producing the anti-inflammatory cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. IL-10 compared with the leukocytes of urban kids. influence of soil on human health
Insulin gives an extra boost to the immune system 8)
Results indicate a relationship between muscular TLR4, p-AMPK and NF-κB content and insulin sensitivity. The study also highlights that in situations of insulin resistance, such as in diabetic subjects, metformin treatment may prevent attenuation of activation of the inflammatory pathway. 9)
Glucose homeostasis, nutrition and infections during critical illness 10)
Adenosine is an important molecule that exerts control on the immune system, by signaling through receptors lying on the surface of immune cells. 11)
An exciting area of recent investigation has arisen from the discovery that SCFA play a role in regulating the immune system and inflammatory response. Early work at the turn of this century had demonstrated the potential role of butyrate in immune regulation when it was shown that butyrate inhibits nuclear factor kappa β (NF-κΒ) activation in macrophages and also inhibits histone deacetylation (HDAc) in acute myeloid leukemia.
Whether SCFA act as a signal to induce tolerance to the host-associated microbiome or directly reduce inflammatory responses remains to be fully elucidated. SCFA do appear able to reduce the responsiveness of lamina propria macrophages to commensal bacteria, via nitric oxide, IL-6, and IL-12 independent of FFAR signaling, to induce tolerance.
Recent data also suggests butyrate suppresses TNF-α, IL-6, and myeloperoxidase activity by preventing NF-κΒ activation in Kupffer cells.12)
Nuclear receptors and ligands
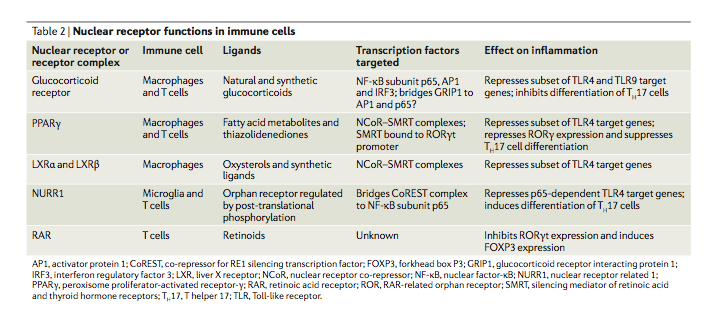
Nuclear receptors are a class of proteins found within the interior of cells that are responsible for sensing the presence of hormones and certain other molecules. A unique property of nuclear receptors which differentiate them from other classes of receptors is their ability to directly interact with and control the expression of genomic DNA. Some of the molecules (or ligands) which bind the nuclear receptor activate (agonize) it and some inactivate (antagonize) it.
It is commonly accepted that most ligands, approximately 95% to 98%, inactivate the nuclear receptors. Since the nuclear receptors play a significant role in the immune response, this factor alone may explain why so many drugs and substances found in food and drink are immunosuppressive.
Because the expression of a large number of genes is regulated by nuclear receptors, ligands that activate these receptors can have profound effects on the organism. Many of these regulated genes are associated with various diseases which explains why the molecular targets of approximately 13% of FDA approved drugs are nuclear receptors.13)
Different cell types have different nuclear receptors. One of the nuclear receptors seen in immune cells is the Vitamin D Receptor (VDR). The VDR has two endogenous or “native” ligands, which are also the two main forms of vitamin D in the human body: 25-hydroxyvitamin D (25-D) and 1,25-dihydroxyvitamin D (1,25-D). Non-native or exogenous ligands can also inactivate or activate a nuclear receptor, depending on its molecular structure.
Ligands compete to dock at nuclear receptors. When is a given kind of ligand such as 25-D as opposed to 1,25-D more likely to bind to the VDR? It depends. 1,25-D tends to be much less common than 25-D – by a factor of 1,000 or more – so it binds to the receptor much more infrequently. A greater concentration of a given molecule can displace competing molecules off the nuclear receptor. Affinity occurs in logarithmic fashion, which is to say that it operates on the basis of a sliding scale. In short, an increase in 1,25-D and a decrease in 25-D can tilt the odds in favor of 1,25-D, and vice versa.
Affinity as well as the question of whether a ligand inactivates or activates a nuclear receptor can all be validated using in silicoExperiment technique performed on computer or via computer emulation. modeling. Although less precise, it is also possible to measure these properties in vitro.
Role of Vitamin D Receptor in innate immunity
Vitamin D/VDR have multiple critical functions in regulating the response to intestinal homeostasis, tight junctions, pathogen invasion, commensal bacterial colonization, antimicrobe peptide secretion, and mucosal defense…. The involvement of Vitamin D/VDR in anti-inflammation and anti-infection represents a newly identified and highly significant activity for VDR.
Jun Sun 14)
When activated by 1,25-D, the Vitamin D Receptor (also called the calcitriol receptor) transcribes thousands of genes.15) It is commonly known that the VDR functions in regulating calcium metabolism.16) It is becoming increasingly clear, however, that the clinically accepted role of the Vitamin D metabolites, that of regulating calcium homeostasis, is just a small subset of the functions actually performed by these hormones.
Transcription of antimicrobial peptides
Other antimicrobial activity of the VDR
Additionally, when the VDR is activated, TLR2 is expressed.19) TLR2 is a receptor, which is expressed on the surface of certain cells and recognizes native or foreign substances, and passes on appropriate signals to the cell and/or the nervous system.
When activated TLR2 allows the immune system to recognize gram-positive bacteria, including Staphylococcus aureus20) 21) Chlamydia pneumoniae22) and Mycoplasma pneumoniae.23) TLR2 also protects from intracellular infections such as Mycobacteria tuberculosis.24)
Antimicrobial peptides
The antimicrobial peptides (AMPs), of which there are hundreds, are families of proteins, which have been called “the body's natural antibiotics,” crucial for both prevention and clearance of infection. AMPs are broad-spectrum, responding to pathogens in a non-specific manner.25)
For example, consider cathelicidin, a protein transcribed by the VDR, which not unlike a Swiss Army knife, has many different functions. Because it can be differentially spliced, the cathelicidin protein itself can respond to a range of very different microbial challenges. In humans, the cathelicidin antimicrobial peptide gene encodes an inactive precursor protein (hCAP18) that is processed to release a 37amino-acid peptide (LL-37) from the C-terminus. LL-37 is susceptible to proteolitic processing by a variety of enzymes, generating many different cathelicidin-derived peptides, each of which has specific targets. For example, LL-37 is generated in response to Staphylococcus aureus, yet LL-37 represents 20% of the cathelicidin-derived peptides, with the smaller peptides being much more abundant and able to target even more diverse microbial forms.26)
AMPs have been documented to kill bacteria and disrupt their function through the following modes of action:
- interfering with metabolism
- targeting cytoplasmic components
- disrupting membranes
- act as chemokines and/or induce chemokine production, which directs traffic of bacteria
Also, AMPs aid in recovery from infection by:
- promoting wound healing
- inhibiting inflammation
In many cases, the exact mechanism by which antimicrobial peptides kill bacteria is unknown. In contrast to many conventional antibiotics including those used by the Marshall Protocol, AMPs appear to be bacteriocidal (a killer of bacteria) instead of bacteriostatic (an inhibitor of bacterial growth).
Two of the more significant families of AMPs are cathelicidin and the beta-defensins. Of these two families, cathelicidin is the most common.
Cathelicidin is a “fire alarm”, generating protective NLRP3-dependent airway epithelial cell inflammatory responses 27)
The full extent by which microbes interfere with AMP expression is the subject of a rapidly growing body of research.28) 29) 30)
Recent research into valuable proteins
Whey proteins and numerous growth factors that regulate insulin secretion, differentiation of intestine epithelium cells, and also tissue restoration, are priceless in stimulation the immune system.
Lactoferrin shows the most comprehensive pro-health properties: antioxidative, anticancer, immune stimulative and even chemopreventive. Also peptides and amino acids formed from casein and whey proteins possess immune stimulative activity. The most valuable proteins, i.e. lactoferrin, immune globulins, lactoperoxidase and lisozyme, together with bioactive peptides, are resistant to pepsin and trypsin activity. This is why they maintain their exceptional biological activity. 31)
Antimicrobial peptides target fungi and viruses
The antimicrobial peptides play a role in mitigating the virulence of the virome and other non-bacterial infectious agents. In addition to its antibacterial activity, alpha-defensin human neutrophil peptide-1 inhibits HIV and influenza virus entry into target cells.32) It diminishes HIV replication and can inactivate cytomegalovirus, herpes simplex virus, vesicular stomatitis virus and adenovirus.33) In addition to killing both gram positive and gram-negative bacteria, human beta-defensins HBD-1, HDB-2, and HBD-3 have also been shown to kill the opportunistic yeast species Candida albicans.34) Cathelicidin also possesses antiviral and antifungal activity.35) 36)
In other words, there is a reason why this group of proteins are named antimicrobial peptides rather than antibacterial peptides.
Unexpected antimicrobial peptides
There are now several examples of substances believed to cause disease, which have since been proven to be part of host defense.
- amyloid beta (amyloid-β) – In a seminal 2010 study, a team of Harvard researchers showed that amyloid beta – the hallmark of Alzheimer's disease – can act as an antimicrobial peptide, having antimicrobial activity against eight common microorganisms, including Streptococcus, Staphylococcus aureus, and Listeria.37) This led study author Rudolph E. Tanzi, PhD to conclude that amyloid beta is “the brain's protector.” However, a 2010 study suggests that toxic levels of amyloid beta “dramatically suppresses VDR expression.” This suggests that overexpression of amyloid beta serves the interests of at least some microbes.38) Read more.
- certain human prion proteins
Evolutionarily conserved
The TLR2/1 and cathelicidin-vitamin D pathway has long played a “powerful force” in protecting the body against infection. This is evidenced by the fact that the Alu short interspersed element (SINE), which transcribes the vitamin D receptor binding element (VDRE), has been evolutionarily conserved for 55-60 million years, but not prior.39) The differences in this pathway between humans/primates and other mammals call into question animal models that try to emulate the vitamin D system and indeed the immune system.
Energy consumption
It has become clear in recent years that pretty much any immune cell in our body undergoes, upon its activation, a metabolic shift resembling the Warburg effect originally described for cancer cells. Immune cells increase glucose consumption and produce a significant portion of ATP by glycolysis ending with lactate even under oxygenated conditions; increased glycolysis is required for the generation of intermediate metabolites associated with the activation of the immune cell.
Increased energy consumption by immune cells requires a metabolic adaptation of the whole organism. During trauma or infection, the organism vitally depends on the immune system, which is therefore privileged in energy/nutrient allocation. According to Rainer Straub [2], insulin resistance caused by pro-inflammatory cytokines is a physiological way of the immune system to usurp energy/nutrients during acute stress from the rest of the organism because immune cells themselves do not become insulin resistant. 40)
Inflammation
Another component of the innate immune response is inflammation, the universal initial response of the organism to any injurious agent.41) Inflammation is a systemic physiological process fundamental for survival.42) The identification of bacteria and other pathogens triggers the release of inflammatory cytokines. These cytokines include interferon-gammaAn inflammatory cytokine which causes extra mast cells to differentiate to monocytes and then to further differentiate into macrophages and dendritic cells. These phagocytes are the most active cells of the immune system and are charged with digesting bacterial pathogens., tumor necrosis factor-alphaA cytokine critical for effective immune surveillance and is required for proper proliferation and function of immune cells. (TNF-alpha), and Nuclear Factor-kappa BA protein that stimulates the release of inflammatory cytokines in response to infection (NF-kappaBA protein that stimulates the release of inflammatory cytokines in response to infection). Cytokines are regulatory proteins, such as the interleukins and lymphokines, that are released by cells of the immune system and act as intercellular mediators in the generation of an immune response. The result is what medicine calls inflammation, which generally leads to an increase in symptoms.
Th1/Th17 inflammation
One key type of inflammation is the Th1/Th17 (T-helper) inflammatory response. In the interests of concision, the Th1/Th17, on this site and others, the Th1/Th17 response is referred to as the Th1 response. This reaction occurs in response to intracellular pathogens, which according to the Marshall Pathogenesis, play a driving force in chronic disease.
All Th1 diseases are marked by an inflammatory response
Before the Human Microbiome Project, scientists couldn't consistently link bacteria to inflammatory diseases. But with the advent of DNA sequencing technology, scientists have detected many of the bacteria capable of generating an inflammatory response. All diseases of unknown etiology are inflammatory diseases.
An inflammatory immune response—one of the body’s primary means to protect against infection—defines multiple established infectious causes of chronic diseases, including some cancers. Inflammation also drives many chronic conditions that are still classified as (noninfectious) autoimmuneA condition or disease thought to arise from an overactive immune response of the body against substances and tissues normally present in the body or immune-mediated (e.g., systemic lupus erythematosus, rheumatoid arthritis, Crohn’s disease). Both [the innate and adaptive immune systems] play critical roles in the pathogenesis of these inflammatory syndromes. Therefore, inflammation is a clear potential link between infectious agents and chronic diseases.
Siobhán M. O'Connor et al. 43)
Th2 inflammation
According to the Marshall Pathogenesis, generally speaking, any activity of the Th2 cytokines in chronic disease is a result of the primary Th1-inducing pathogens.
Many palliative therapies interfere with inflammation
Related article: Palliative vs. curative treatments
While inflammation is associated with disease, inflammation often serves an invaluable role as the immune system fights off chronic pathogens. Numerous medications artificially suppress inflammation including anti-TNF drugsDrugs which interfere with the body's production of TNF-alpha - a cytokine necessary for recovery from infection, interferon, corticosteroidsA first-line treatment for a number of diseases. Corticosteroids work by slowing the innate immune response. This provides some patients with temporary symptom palliation but exacerbates the disease over the long-term by allowing chronic pathogens to proliferate., antifungals, and anti-pyreutics. While interfering with the inflammatory response typically reduces immunopathologyA temporary increase in disease symptoms experienced by Marshall Protocol patients that results from the release of cytokines and endotoxins as disease-causing bacteria are killed. and makes a patient feel less symptomatic in the near term, doing so allows the bacteria which cause chronic disease to proliferate.
The release of cytokines appears to be essential for recovery after an infection. One study found that the cytokine TNF-alpha – which is blocked by anti-TNF drugs – is necessary for the proper expression of acquired specific resistance following infection with Mycobacterium tuberculosis.44) 45) 46) Another effect of the use of TNF blockers is to break or reduce the formation of granuloma, one of the body's mechanisms to control bacterial pathogens.47)
Commensal microbes
Main article: Probiotics and commensal bacteria
The host innate immune defense system is highly active in healthy tissue.48) Commensal bacteria can activate innate immune responses.49) 50)
Immune suppression
The surprising finding of science is that home and hospital environments can be less supportive of human health than the outdoors. exposure to environmental microbes helps protect against allergies and other inflammatory diseases 51) Exposure to environmental microbes helps protect against allergies and other inflammatory diseases
Environmental pollution, both visible and invisible, many palliative medications, and increasingly this century the cellular level radiation from wifi devices, are interfering with the capacity of the innate immune system to create the defensive molecules necessary to control intracellular infections
[PMID: 19400860] [DOI: 10.1111/j.1600-0463.2009.02456.x]
[PMID: 20736230] [PMCID: 2945184] [DOI: 10.1101/gr.107920.110]
[PMID: 16002434] [DOI: 10.1210/me.2005-0106]
[PMID: 11807545] [DOI: 10.1038/415389a]
[PMID: 18689389] [DOI: 10.1093/ajcn/88.2.491S]
[PMID: 14698282] [DOI: 10.1016/j.it.2003.11.003]
[PMID: 9540269] [DOI: 10.1016/s0167-5699(97)01204-8]
[PMID: 30174303] [DOI: 10.1016/j.cmet.2018.08.003]
[PMID: 28791487] [DOI: 10.1007/s00592-017-1027-5]
[PMID: 28082192] [DOI: 10.1016/j.cmi.2016.12.033]
[PMID: 27557887] [PMCID: 5124006] [DOI: 10.1007/s11302-016-9529-0]
[PMID: 26963409] [PMCID: 4939913] [DOI: 10.1080/19490976.2015.1134082]
[PMID: 17139284] [DOI: 10.1038/nrd2199]
[PMID: 20639756] [PMCID: 2955835] [DOI: 10.1097/MOG.0b013e32833d4b9f]
[PMID: 11500311] [DOI: 10.1152/ajpendo.2001.281.3.E558]
[PMID: 15322146] [DOI: 10.4049/jimmunol.173.5.2909]
[PMID: 15985530] [DOI: 10.1096/fj.04-3284com]
[PMID: 17290304] [PMCID: 1784003] [DOI: 10.1172/JCI30142]
[PMID: 11067888] [DOI: 10.4049/jimmunol.165.10.5392]
[PMID: 15731086] [PMCID: 1064969] [DOI: 10.1128/IAI.73.3.1847-1851.2005]
[PMID: 17145941] [PMCID: 1828523] [DOI: 10.1128/IAI.01386-06]
[PMID: 15843573] [DOI: 10.4049/jimmunol.174.9.5713]
[PMID: 19442756] [DOI: 10.1016/j.micinf.2009.04.025]
[PMID: 19817855] [DOI: 10.1111/j.1742-4658.2009.07360.x]
[PMID: 30978238] [PMCID: 6481867] [DOI: 10.1371/journal.ppat.1007694]
[PMID: 18717821] [DOI: 10.1111/j.1462-5822.2008.01227.x]
[PMID: 15695583] [PMCID: 549001] [DOI: 10.1073/pnas.0409588102]
[PMID: 15953032] [DOI: 10.1111/j.1462-5822.2005.00530.x]
[PMID: 24720113]
[PMID: 18809937] [PMCID: 2612179] [DOI: 10.1128/AAC.00470-08]
[PMID: 16630942] [PMCID: 2727734] [DOI: 10.1016/j.jaci.2005.12.1345]
[PMID: 17023499] [DOI: 10.1093/jac/dkl382]
[PMID: 20209079] [PMCID: 2831066] [DOI: 10.1371/journal.pone.0009505]
[PMID: 20966550] [DOI: 10.3233/JAD-2010-101377]
[PMID: 19607716] [PMCID: 2716374] [DOI: 10.1186/1471-2164-10-321]
[PMID: 26427038] [PMCID: 4741691] [DOI: 10.18632/oncotarget.4685]
[PMID: 20540037]
[PMID: 20388071] [DOI: 10.2174/138161210790883840]
[PMID: 16836820] [PMCID: 3291059] [DOI: 10.3201/eid1207.060037]
[PMID: 18544042] [PMCID: 2612548] [DOI: 10.1111/j.1365-2567.2008.02865.x]
[PMID: 12852719]
[PMID: 15216471] [DOI: 10.1086/421522]
[PMID: 16322600] [PMCID: 2713334] [DOI: 10.1513/pats.200508-091JS]
[PMID: 20514045] [DOI: 10.1038/nrmicro2337]
[PMID: 19161412] [DOI: 10.1111/j.1600-065X.2008.00701.x]
[PMID: 12960260] [DOI: 10.1189/jlb.0203082]
[PMID: 19809501] [PMCID: 2752989] [DOI: 10.1371/journal.pone.0007358]
[PMID: 19000576] [DOI: 10.1016/j.jaci.2008.10.002]
[PMID: 20414208] [DOI: 10.1038/nri2748]