Related article: In vitro studies

Table of Contents
Animal models
Animal models have long been used to test theories of and therapies for chronic inflammatory diseases. Among the different animal models, the rodent may be the most widely used. Because they are quick to reach sexual maturity and are easily kept and bred in captivity, rodents have been praised as prime experimental subjects.
Unfortunately, rodents and humans are strikingly different in a number of important ways, especially with regard to their immune system. Animal models of disease are not a valid mode for emulating chronic disease, including cancers. For this reason, there have yet to be any major breathroughs from murine (mouse) work; and there may never be.
Widespread use of rodents in human disease research
The use of rodents is widespread among researchers: mice have been used for models of cancer and any number of chronic diseases. So widely accepted is the use of mouse models that it has become difficult to identify which contributions are based on mouse models and which contributions are based on human models.
Unraveling the intricacies of human [Vitamin] D metabolism is often made extremely difficult by the intermingling of murine [mouse] and human biologies in the literature.
Trevor Marshall, PhD
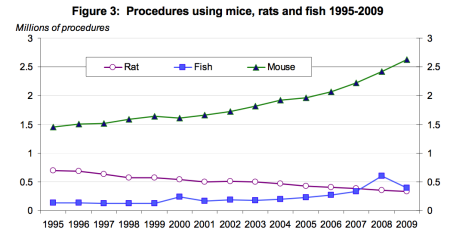
The Methuselah Foundation, an institute focused on human longevity, funds the Methuselah Mouse Prize or MPrize, a multi-million dollar award designed to hasten the research into effective life extension interventions. The MPrize awards researchers who stretch the lifespan of mice to unprecedented lengths. Even if the prize were awarded, there is legitimate reason to be concerned that the advances in curtailing murine diseases of the aging could not be successfully applied to humans.
Inconsistency among competing mouse models
It is sometimes the case that different rodent models lead an observer to competing conclusions.
To cite but one example, while the FDA currently accepts carcinogenicity studies of pharmaceutical drugs based on murine models, competing mouse models often conflict with each other. For example, during the FDA acceptance testing (2002) of the ARBA drug which is an angiotensin receptor blocker. One of the ARBs is olmesartan (Benicar). Not all ARBs activate the Vitamin D Receptor. olmesartan (Benicar)Medication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor., possible carcinogenicity observed in hamsters was not able to be duplicated in rats or in transgenic mice.
Failing to consistently account for microbes
Often, the implementation a mouse model will produce unexpected results, because it does not account for the role of microbes in causing disease, especially when it comes to microbes infecting immune cells.
How can one hypothesize a human environment where some classes of immune cells are infected and some are not? You can create those conditions in a lab, but not in-vivo.
Trevor Marshall, PhD
As described in the magazine, The Scientist, Stephen Miller from Northwestern University attempted to recreate virus-triggered demyelinating diseases such as multiple sclerosis in mice.
Working with these transgenic mice, he noticed that animals that normally develop autoimmunity did not do so when reared in a supersterile environment. Although the mice were engineered to express lots of T cells specific to myelin, the T cells were not attacking the central nervous system. The missing factor was their gut microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms.. “Now the big question is,” Wekerle says, “which bacteria in the gut are responsible for activation and triggering of the disease? Could it also be that in people with MS, the initial step has been in the gut?”
Differences between human and rodent immune function
Related article: Innate immune response and Th1 inflammation
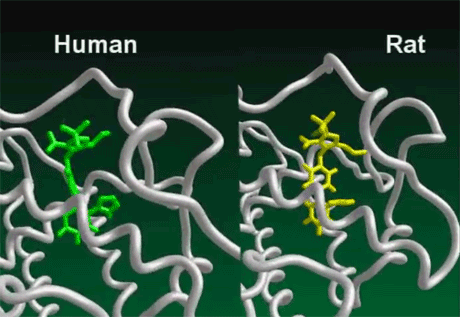
Multiple studies have demonstrated that the immune systems of rats and humans are inherently different. Molecular modeling research has revealed that the activity of the human innate immune system is controlled by the Vitamin D Receptor. In humans, the Vitamin D Receptor performs several fundamental roles. Not only does it control the activity of the innate immune system, but it transcribes 913 genes, and possibly more. It also controls the production of many of the antimicrobial peptidesBody’s naturally produced broad-spectrum antibacterials which target pathogens.. These peptides kill bacteria, viruses, and fungi by a variety of mechanisms, including disrupting membranes, interfering with metabolism, and targeting components of the machinery inside the cell.1)2)
In contrast, the rat innate immune system is not controlled by the Vitamin D Receptor. It depends on a cascade of nitric oxide (an important signaling molecule) that functions in a manner yet to be fully understood. Rats do have Vitamin D Receptors, but they transcribe different genes than the human VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response.. By using molecular modeling software, researchers at McGill University in Canada found many differences in the genes targeted by the rat and human Vitamin D Receptors. For example, the gene encoding a calcium binding protein called osteocalcin is “robustly” transcribed by the VDR in humans, but not in mice. In what proves to be a fundamental difference between mice and men, Manisha Brahmachary and team recently determined that the rat Vitamin D Receptor does not express the cathelicidin Family of antimicrobial peptides found primarily in immune cells and transcribed by the Vitamin D Receptor. antimicrobial peptides (AMPs)–marking an important difference in the way the two species kill invading pathogens.3) This means that rats and humans respond differently to molecules or drugs that affect the VDR and subsequently the innate immune system.
According to Mestas et al., there are significant differences between the way the rat and human immune systems develop, which affect “activation, and response to challenge, in both the innate and adaptive arms [of the immune system].” Such differences should not be surprising, as rats and humans diverged somewhere between 65 and 75 million years ago and differ hugely in both size and lifespan. According to Mestas, “They have also evolved in quite different ecological niches where widely different pathogenic challenges need to be met–after all, most of us do not live with our heads a half-inch off the ground.” Consequently, he argues that, “There has been a tendency to ignore differences and in many cases, perhaps, make the assumption that what is true in mice is necessarily true in humans. By making such assumptions we run the risk of overlooking vital aspects of human immunology that do not occur, or cannot be modeled, in mice.”4)
Validity of murine models
While there are certainly exceptions,5) generally speaking the murine immune system tends not to be a valid approximation of the human immune system.
In a systematic review comparing the treatment effects of animal experiments and clinical trials, Perel et al. review a number of examples where interventions used on animals are not duplicated in humans. These include head injury, antifibrinolytics in hemorrhage, thrombolysis in acute ischaemic stroke, tirilazad in acute ischaemic stroke, antenatal corticosteroidsA first-line treatment for a number of diseases. Corticosteroids work by slowing the innate immune response. This provides some patients with temporary symptom palliation but exacerbates the disease over the long-term by allowing chronic pathogens to proliferate. to prevent neonatal respiratory distress syndrome, and bisphosphonates to treat osteoporosis.
The evidence against the validity of many animal models is prompting some researchers to question the validity of animal studies.
Discordance between animal and human studies may be due to bias or to the failure of animal models to mimic clinical disease adequately.
Pablo Perel et al. 6)
Read more
Related publications and presentations
[PMID: 17290304] [PMCID: 1784003] [DOI: 10.1172/JCI30142]
[PMID: 15322146] [DOI: 10.4049/jimmunol.173.5.2909]
[PMID: 14978070] [DOI: 10.4049/jimmunol.172.5.2731]
[PMID: 18364394] [PMCID: 2278204] [DOI: 10.1073/pnas.0711558105]
[PMID: 17175568] [PMCID: 1781970] [DOI: 10.1136/bmj.39048.407928.BE]


