Related article: Th1 Spectrum Disorder

Table of Contents
Th1 Spectrum Disorder
Th1 Spectrum DisorderThe overlap of different disease symptoms in different patients with similar diagnoses - caused by the fact that any one bacterial species can contribute to numerous disease states. refers to the group of chronic inflammatory diseases, which are hypothesized to be caused by the Th1 pathogensThe community of bacterial pathogens which cause chronic inflammatory disease - one which almost certainly includes multiple species and bacterial forms., a microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms. of bacteria which include L-formDifficult-to-culture bacteria that lack a cell wall and are not detectable by traditional culturing processes. Sometimes referred to as cell wall deficient bacteria., biofilm A structured community of microorganisms encapsulated within a self-developed protective matrix and living together., and intracellular bacterial forms. Although the exact species and forms of bacteria, as well as the location and extent of the infection, vary between one patient suffering from chronic disease and the next, the disease process is common: bacterial pathogens persist and reproduce by disabling the innate immune responseThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease..
Although patients who become infected with the Th1 pathogens are given a variety of diagnoses, there are often no clear cut distinctions between one disease and the next. Rather, symptoms frequently overlap creating a spectrum of illness in which diseases are more connected to one another than mutually exclusive disease states.
The evidence that chronic disease is ultimately a spectrum disorder with a common cause includes:
- comorbidity of inflammatory diseases overlap observed between patients suffering distinctly defined diseases
- the infrequency with which patients suffer from just a single disease or condition
- failure of diagnostic compartmentalization: the frequent difficulty doctors have in clearly and definitively diagnosing a patient
Conflict in theories
Traditionally, diseases are understood to be discrete and have their own respective and distinct pathologies. This theory of disease has been advanced by researchers intent upon pinpointing the human genes which they theorize cause disease. The existence of a vast network of clinical specialists and sub-specialists only reinforces this idea.
According to the Marshall PathogenesisA description for how chronic inflammatory diseases originate and develop., a range of chronic diseases is caused by a common etiology or disease process. Patients accumulate Th1 pathogens, which proliferate by disabling the Vitamin D ReceptorA nuclear receptor located throughout the body that plays a key role in the innate immune response. and consequently weakening the innate immune response.
Comorbidity of inflammatory diseases
One of the striking features of a variety of neuropsychiatric diseases (e.g., affective disorders) is their variance, with differences observed across individuals in terms of their susceptibility, in the combination of systems that are disturbed, and in the therapeutic and adverse responses to various medications…. The microbiome [may represent] a source of this observed variance.
A. Gonzalez et al.1)
When the Th1 pathogens compromise the immune response, they make it easier for other types of bacteria in other locations to infect the body as well. This phenomenon is known as comorbidity. Although a comorbid condition is traditionally understood to be unrelated to the underlying condition, the sheer number of common comorbidities points to a common pathology. Indeed, a 2012 Dutch analysis of patients showed that disease pairs occurred more frequently than would be expected if diseases had been independent.2)
Epidemiological research may have its share of liabilities, but one contribution it has made is in demonstrating the strong connections between seemingly disparate diseases as evidenced by the number of patients who share diagnoses with two or more “unrelated” disease processes.
The following wheel shows how truly related chronic diseases are. Each “spoke” represents a published study which has demonstrated a significant statistical relationship between patients suffering from one disease and the next.
Please note that some of the disease names in the following paragraph are links to articles discussing those diseases in further detail.
allergies 3) alopecia areata 4) 5) Alzheimer's disease & dementia 6) 7) ankylosing spondylitis 8) 9) 10) anorexia nervosa 11) anxiety disorders 12) 13) 14) 15) 16) 17) 18) 19) 20) arthritis 21) 22) 23) 24) 25) 26) 27) asthma 28) 29) 30) 31) 32) 33) 34) 35) 36) 37) 38) 39) bipolar disease 40) 41) 42) 43) cancer 44) 45) 46) 47) cardiac disease 48) 49) 50) 51) 52) 53) 54) 55) 56) 57) cardiovascular disease 58) 59) 60) 61) 62) celiac disease 63) 64) 65) 66) chronic fatigue syndrome 67) 68) 69) chronic obstructive pulmonary disease 70) depression 71) 72) 73) 74) 75) 76) 77) 78) 79) 80) 81) 82) 83) 84) 85) 86) 87) diabetes 88) 89) 90) 91) 92) 93) 94) diabetes, type1 95) 96) 97) diabetes, type2 98) fibromyalgia 99) 100) Guillain-Barré syndrome 101) 102) hypertension 103) 104) inflammatory bowel disease (Crohn's disease and ulcerative colitis) 105) 106) 107) 108) 109) 110) 111) 112) 113) 114) 115) 116) 117) 118) lupus 119) 120) 121) 122) 123) multiple chemical sensitivity 124) multiple sclerosis 125) 126) 127) 128) 129) 130) 131) myasthenia gravis 132) obesity 133) 134) 135) 136) 137) obsessive compulsive disorder 138) 139) 140) 141) 142) osteoporosis 143) 144) 145) 146) Parkinson's disease 147) 148) 149) 150) periodontal disease 151) 152) pernicious anemia 153) psoriasis 154) 155) 156) 157) 158) rheumatoid arthritis 159) 160) 161) sarcoidosis 162) 163) schizophrenia 164) 165) 166) scleroderma 167) Sjogren's syndrome 168) 169) 170) 171) thyroiditis (Graves' disease and Hashimoto's thyroiditis) 172) 173) 174) 175) 176) 177) 178) uveitis 179) 180) vitiligo 181)
Multimorbidity
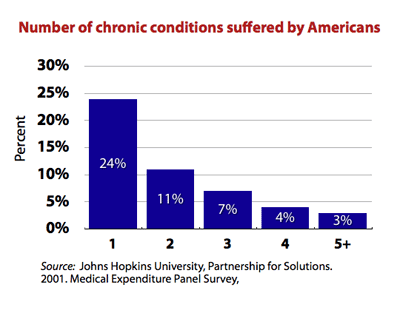
According to a 2012 JAMA paper, the most common chronic condition experienced by adults is multimorbidity, the coexistence of multiple chronic diseases or conditions.182) In patients with coronary disease, for example, it is the sole condition in only 17% of cases.183) Almost 3 in 4 individuals aged 65 years and older have multiple chronic conditions, as do 1 in 4 adults younger than 65 years who receive health care.184) Adults with multiple chronic conditions are the major users of health care services at all adult ages, and account for more than two-thirds of health care spending.185)
To control for confounding variables, researchers often exclude patients with more than one condition from research studies even though they represent the majority of patients.186) Jeste et al made this observation in patients with schizophrenia187) and there's no reason to think it's any different with other patient groups.
A recent survey of Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. patients, discussed in Amy Proal's presentation at Congress on Autoimmunity, showed that of those with Hashimoto's thyroiditis, only 8% had been diagnosed with Hashimoto's alone.
Metabolic syndrome
Impact of 25-hydroxyvitamin D, free and bioavailable fractions of vitamin D, and vitamin D binding protein levels on metabolic syndrome components 188)
Intestinal barrier
Zonulin and its regulation of intestinal barrier function: the biological door to inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue., autoimmunity, and cancer. 189)
The two major triggers of zonulin release that have been described so far are bacteria and gliadin. It is well described that many enteric pathogens are able to produce enterotoxins that affect the intestinal tight junction of the host. 190)
The challenge of diagnosing diseases caused by bacteria
Many doctors are reluctant to say that chronic diseases are caused by bacterial pathogens and for a couple of reasons.
No consistent symptom presentation
Medicine has difficulty diagnosing disorders where there is no consistently identified anatomic abnormality or documented metabolic/physiological dysfunction.191)
Diagnoses are driven by perceived therapeutic options
What a clinician thinks causes many of the ill-defined chronic diseases may in fact be shaped by available treatment options for that disease.
Consider dentists. Most dentists will readily concede that bacteria cause plaque and tooth decay. The fact that a dentist can employ a therapy against plaque (in this case, manually removing plaque) clearly shapes their opinion about the etiology of the disease. That the intervention is at least temporarily effective also has something to do with it, but perhaps not as much as most people might imagine.
When it comes to lethargy or exercise intolerance or difficulty breathing or any number of other symptoms of disease, the explanation for the disease's etiology is often driven by the available mainstream treatment options, of which very few are effective. These options are themselves highly influenced by pharmaceutical companies which have a vested interest in selling a drug or treatment. In their drive to differentiate themselves and their product, these companies will overemphasize the distinctions among diseases when there may be no such fundamental differences.
False assurance of diagnostic compartmentalization
Another challenge relates to how diseases are segmented into categories even when the nature of the diseases themselves don't warrant such fine-graded distinctions.
One who pores through the articles of a medical textbook could easily form the impression that diseases are discrete, well-defined and mutually exclusive. The reality is that the nature of illness is such that diagnosis is often inexact. Over the past few decades, the sensitivity and specificity of diagnostic tests has, in many cases, increased dramatically. Yet, neither precision nor accuracy is useful when two test results for a patient suggest conflicting diagnoses.
To resolve this ambiguity, epidemiologists have developed a kind of stop-gap measure: rubrics - many of them “evidence-based” - for diagnosing disease. A rubric is a checklist of sorts. Doctors who diagnose according to a rubric look to see if a patient has at least a certain number of classical symptoms - say, eight of the twelve symptoms listed. Given that the vagaries of any one chronic disease are determined by patients' unique pea soup, that is, their particular mix of Th1 pathogens, the traditional methods for diagnosis leave something to be desired.
Patients presenting with prototypical cases of a given disease tend to be the exception rather than the rule. They may have some of the classical symptoms of a given disease but not others. Also, patients may have symptoms that are unique to a different disease. Patients with symptoms of chronic disease could present five different doctors with the same set of symptoms and get five different diagnoses, and many have!
Excessive testing
In the face of uncertainty and ambiguity, a clinician's natural response might be to order a battery of tests. After all, the more information clinicians obtain, the more confidence they have in the validity of their diagnoses, even when such confidence may not be justified on the basis of the information obtained.
In his paper, “Our stubborn quest for diagnostic certainty: a cause of excessive testing,” JP Kassirer writes:
Absolute certainty in diagnosis is unattainable, no matter how much information we gather, how many observations we make, or how many tests we perform. Our task is not to attain certainty, but rather to reduce the level of diagnostic uncertainty enough to make optimal therapeutic decisions…. We continue to test excessively, partly because of our discomfort with uncertainty.
Jerome Kassirer, MD 192)
In practice, as one approaches diagnostic certainty the useful information returned by diagnostic tests and observations approaches zero.193)
A 2011 article in the Daily Beast showed how some common tests and procedures may do more harm than good.
A single diagnosis for chronic inflammatory disease
Given that all of the so-called autoimmune diseases and chronic infections are a variation of the Th1 inflammatory process, neither a specific diagnostic label or identification of specific pathogens is needed to begin the Marshall Protocol (MP). With the MP, patients identify Th1 inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue. with simple blood tests and then confirm the presence of occult microbes with a therapeutic probeA brief trial of the Marshall Protocol to see if it will generate an immunopathological response. The "gold standard" for testing whether a patient is a good candidate for the MP.. As treatment continues, the presence of the immune system reactions confirms the continuing efficacy of treatment. And finally, symptom resolution, absence of immune system reactions and normal blood work indicate recovery.
Where the MP is unique is that I set out to kill pathogens which have never been fully identified, whose exact nature is still largely unknown. I did this based on an understanding of the pathogens' biochemical effects on the body, and the consequent understanding of how they must therefore be exerting that effect…. We have focused on the commonalities, rather than the differences, between immune disease syndromes, and this tends to make it easier to distinguish the forest from the trees.
Trevor Marshall, PhD
A corollary of this principle is that attempts to compare one patient's disease with another's may be futile. Certainly there is a great deal of variability in the location and severity of infection and the corresponding symptoms, but ultimately there is no fundamental difference between the origin of patients' disease states and there is, therefore, no significant difference in their ultimate recovery trajectories.
Health and disease is a continuum
…because the women are all healthy when they enroll [then any disease can be detected as they continue sampling from that time].
Claire Fraser-Liggett, Director of the Institute for Genome Sciences at the University of Maryland, BBC Radio 4 program about the Human Microbiome
It is common practice to assign one group of patients participating in a controlled trial to be the “healthy control group.” While researchers are apt to make a hard distinction between health and disease, this dichotomy is contrary to what we know about successive infection.
The process of successive infection does not just occur in sick people or people who are symptomatic. In healthy subjects, subclinical infection is not the exception, but the rule. For example:
- 30% of healthy people are carriers of the pathogen Staphylococcus aureus.194)
- A 2011 pyrosequencing study looked at 16S rDNA amplicons of eight culture-negative healthy female urine specimens.195) The study found a significant amount of sequences belonging to bacteria with a known pathogenic potential.
From even before birth, every human is constantly acquiring new microbes as demonstrated in several studies196) by Rob Knight and Jeffrey Gordon's team. After sequencing the microbiome of two individuals at four body sites over 396 timepoints, the group essentially concluded that the notion of a “core microbiome” is overblown.
We find that despite stable differences between body sites and individuals, there is pronounced variability in an individual's microbiota across months, weeks and even days. Additionally, only a small fraction of the total taxa found within a single body site appear to be present across all time points, suggesting that no core temporal microbiome exists at high abundance (although some microbes may be present but drop below the detection threshold). Many more taxa appear to be persistent but non-permanent community members.
J. Gregory Caporaso et al. 197)
Clearly, the microbiota can be affected in any number of ways that are not picked up by the relatively crude tests that we use to measure “health.” Further, patients will carry pathogenic elements of their microbiota without symptoms to show for it – at least initially.
The variability of patients' responses – both between control and experimental groups, and among an experimental group (some subjects get a side effect, some don't) – may ultimately be a testament to the unique nature of each person's microbiota.
Because everyone person's microbiota is unique, it may be overly simplistic, if not naive, to say there is such a thing as a person who is truly healthy.
[PMID: 21485746] [PMCID: 3139398] [DOI: 10.31887/DCNS.2011.13.1/agonzalez]
[PMID: 22935268] [PMCID: 3490727] [DOI: 10.1186/1471-2458-12-715]
[PMID: 9042070] [DOI: 10.1016/s0091-6749(97)70126-x]
[PMID: 7883407] [DOI: 10.1111/j.1365-4362.1994.tb01018.x]
[PMID: 17582741] [PMCID: 2717015] [DOI: 10.1016/j.jaut.2007.05.002]
[PMID: 10479741]
[PMID: 18367704] [DOI: 10.1212/01.wnl.0000306313.89165.ef]
[PMID: 16981296]
[PMID: 16143148] [DOI: 10.1053/j.gastro.2005.07.042]
[PMID: 18008072] [DOI: 10.1007/s00296-007-0483-6]
[PMID: 15569892] [DOI: 10.1176/appi.ajp.161.12.2215]
[PMID: 8818377] [DOI: 10.1192/bjp.169.1.101]
[PMID: 18774427] [DOI: 10.1016/j.genhosppsych.2008.04.010]
[PMID: 9361174] [DOI: 10.3109/00365529709011218]
[PMID: 9707157]
[PMID: 12011605] [DOI: 10.1097/00005053-200205000-00001]
[PMID: 11391746] [DOI: 10.1002/mds.1099]
[PMID: 18021889] [DOI: 10.1016/j.clindermatol.2007.08.006]
[PMID: 16143122] [DOI: 10.1053/j.gastro.2005.06.021]
[PMID: 16478843] [DOI: 10.1378/chest.129.2.285]
[PMID: 10599323] [DOI: 10.1007/978-1-4615-4857-7_8]
[PMID: 18582390] [PMCID: 2474682] [DOI: 10.1186/1471-2458-8-221]
[PMID: 17437504] [DOI: 10.1111/j.1572-0241.2007.01215.x]
[PMID: 14706414] [DOI: 10.1016/s0163-8343(03)00071-9]
[PMID: 16491113] [DOI: 10.1038/sj.ijo.0803215]
[PMID: 9856429] [DOI: 10.1001/archpedi.152.12.1197]
[PMID: 12720509] [DOI: 10.5694/j.1326-5377.2003.tb05408.x]
[PMID: 9387083] [DOI: 10.1016/s0165-0327(97)00075-x]
[PMID: 15163249] [DOI: 10.4088/jcp.v65n0507]
[PMID: 12361669] [DOI: 10.1016/s0006-3223(02)01472-5]
[PMID: 16393226] [DOI: 10.1111/j.1572-0241.2005.00287.x]
[PMID: 16338246] [DOI: 10.1016/j.ahj.2005.02.007]
[PMID: 15633853] [DOI: 10.1177/070674370404901106]
[PMID: 11404827] [DOI: 10.1053/sarh.2001.23498]
[PMID: 18453370] [PMCID: 2645334] [DOI: 10.1513/pats.200709-148ET]
[PMID: 17470018] [DOI: 10.1902/jop.2007.060301]
[PMID: 16476152] [DOI: 10.1080/j.1440-1614.2006.01781.x]
[PMID: 17383491] [DOI: 10.1016/j.jpsychores.2006.12.009]
[PMID: 9097215] [DOI: 10.1002/(sici)1099-1166(199702)12:2<219::aid-gps581>3.0.co;2-e]
[PMID: 16371403] [PMCID: 1326926] [DOI: 10.1136/bmj.38678.389583.7C]
[PMID: 17053448] [DOI: 10.1097/01.mog.0000245543.72537.9e]
[PMID: 10647761] [DOI: 10.1001/archinte.160.2.221]
[PMID: 12391767] [DOI: 10.1097/00043764-200210000-00006]
[PMID: 8268331] [DOI: 10.1016/0006-3223(93)90237-8]
[PMID: 8444530] [DOI: 10.1111/j.1365-4362.1993.tb02790.x]
[PMID: 15111522] [DOI: 10.2337/diacare.27.5.1066]
[PMID: 15745840] [DOI: 10.1016/j.jdiacomp.2004.08.003]
[PMID: 17206706] [DOI: 10.1002/ibd.20062]
[PMID: 2027060] [DOI: 10.1902/jop.1991.62.2.123]
[PMID: 11320574]
[PMID: 10943233] [DOI: 10.4065/75.8.802]
[PMID: 15247180] [PMCID: 1774134] [DOI: 10.1136/gut.2003.036657]
[PMID: 2923094] [DOI: 10.1093/ajcp/91.3.259]
[PMID: 16278288] [DOI: 10.1093/rheumatology/kei079]
[PMID: 8809450]
[PMID: 16280086] [PMCID: 1308850] [DOI: 10.1186/1740-2557-2-9]
[PMID: 10489063] [DOI: 10.1212/wnl.53.4.883]
[PMID: 1565240] [DOI: 10.1212/wnl.42.4.845]
[PMID: 2136584] [DOI: 10.1002/ana.410270522]
[PMID: 10758256] [DOI: 10.1016/s0022-3956(99)00048-5]
[PMID: 22797447] [PMCID: 4083627] [DOI: 10.1001/jama.2012.5265]
[PMID: 17848649] [DOI: 10.1001/jama.298.10.1160-b]
[PMID: 8873293] [DOI: 10.1093/schbul/22.3.413]
[PMID: 28721141] [PMCID: 5510509] [DOI: 10.5114/aoms.2016.58594]
[PMID: 28123927] [PMCID: 5214347] [DOI: 10.1080/21688370.2016.1251384]
[PMID: 12685682]
[PMID: 2497349] [DOI: 10.1056/NEJM198906013202211]
[PMID: 1901611]
[PMID: 21297670] [DOI: 10.1038/nrmicro2508]
[PMID: 22047020] [PMCID: 3228714] [DOI: 10.1186/1471-2180-11-244]
[PMID: 20441597] [PMCID: 2898070] [DOI: 10.1186/gb-2010-11-5-210]
[PMID: 21624126] [PMCID: 3271711] [DOI: 10.1186/gb-2011-12-5-r50]
[PMID: 20046873] [PMCID: 2795195] [DOI: 10.1371/journal.pone.0008540]
[PMID: 3098977] [DOI: 10.1099/00222615-22-4-335]
[PMID: 20631792] [PMCID: 2919852] [DOI: 10.1038/nature09199]
[PMID: 20840217] [DOI: 10.1111/j.1574-6941.2010.00908.x]