Related article: Health maintenance and Olmesartan

Table of Contents
Science behind olmesartan (Benicar)
Patients on the Marshall Protocol (MP) take olmesartan (Benicar)Medication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor., a drug whose actions are well known, every six hours. In general, olmesartanMedication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor. Also known by the trade name Benicar. tends to be prescribed for its antihypertensive properties due to the fact that is an angiotensin receptor blocker. A growing body of research supports the use of olmesartan as a part of a curative therapy for chronic disease.
For the purposes of the MP, olmesartan has two primary actions: it reduces inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue. by blocking the Nuclear Factor-kappaB cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. pathway and it is an agonist of the Vitamin D ReceptorA nuclear receptor located throughout the body that plays a key role in the innate immune response. (VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response.). As a VDR agonistA substance such as olmesartan (Benicar) or 1,25-D which activates the Vitamin D Receptor and transcribes the genes necessary for a proper innate immune response., olmesartan uniquely activates the innate immune responseThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease.. Research supports the safety of the doses used by MP patients.
Olmesartan has minimal interactions with other drugs and is one of the safest drugs on the market.
Safety of olmesartan
Main article: Olmesartan safety
Ample research supports the fact that olmesartan is one of the safest and has the most gentle side effects profiles of almost any drug on the market.
For those with gastric issues, it is recommended that the pill be crushed and mixed with applesauce or similar to reduce impact on gastric lining.
Potent anti-inflammatory
Olmesartan is classified as an Angiotensin II Receptor Blocking (ARBA drug which is an angiotensin receptor blocker. One of the ARBs is olmesartan (Benicar). Not all ARBs activate the Vitamin D Receptor.) drug. When olmesartan binds and blocks the Angiotensin Receptor, it prevents fibrotic tissue from forming and decreases levels of Nuclear Factor Kappa B, a protein that stimulates the release of inflammatory cytokinesAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. - proteins that generate pain and fatigue. These cytokines include interferon gamma and TNF-alphaA cytokine critical for effective immune surveillance and is required for proper proliferation and function of immune cells.. The drop in cytokines results in less inflammation and oxidative stress. As inflammation drops, the antibiotics can also perfuse the tissues more effectively.
Working at a higher level than the cytokines or chemokines, olmesartan stops TNF-alpha from being released from the macrophages. It also blocks the other cytokines released during an inflammatory reaction. It does not bind to just any TNF-alpha floating around so it doesn't interfere with the function of the immune system like the TNF-antagonists such as Remicade and Enbrel.
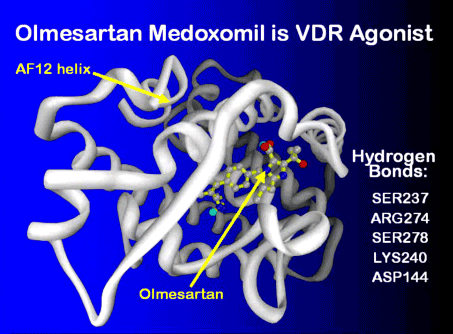
The olmesartan can make patients feel better, allowing them to more easily tolerate the increase in symptoms generated by bacterial die-off. In fact, if patients feel that their immunopathologyA temporary increase in disease symptoms experienced by Marshall Protocol patients that results from the release of cytokines and endotoxins as disease-causing bacteria are killed. is too strong, they can take extra olmesartan in order to help palliate the inflammatory response.
Though described below as an agent which stimulates the immune response, olmesartan may also reduce inflammation through activating the VDR:
The involvement of Vitamin D/VDR in anti-inflammation and anti-infection represents a newly identified and highly significant activity for VDR.
Jun Sun 1)
Vitamin D Receptor agonist
The VDR has been praised as an attractive target for modulating inflammation in autoimmune disease.
Vitamin D receptor (VDR) agonists are well known for their capacity to control calcium metabolism and to regulate growth and differentiation of many cell types. More recently, it has become clear that VDR agonists possess immunoregulatory properties and, in particular, pronounced pro-tolerogenic activities…. These mechanisms of action can explain some of the immunoregulatory properties of VDR agonists in the treatment of Th1-mediated autoimmune diseases, but may also represent a physiologic element in the VDR-mediated regulation of innate and adaptive immune responses.
L. Adorini, from paper “Intervention in autoimmunity: the potential of vitamin D receptor agonists” 2)
Olmesartan's action as VDR agonist makes it the critical component of the Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis.. This is evident in patients taking antibiotics who do not experience immunopathology until they take olmesartan. For this reason, olmesartan meets the definition of an antibacterial.
To us, olmesartan is not a “medication.” It is a method of turning-on your body’s Vitamin D Receptor. This is a key part of the immune system, and transcribes over 1,000 genes which affect body processes from calcium homeostasis to cancer metastasis.
Trevor Marshall, PhD
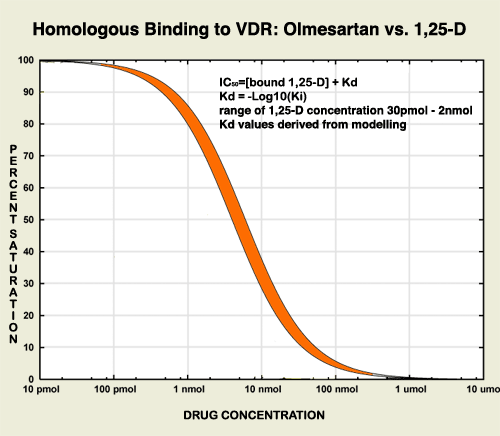
The ribosome blockades initiated by the Marshall Protocol antibiotics weaken the Th1 pathogensThe community of bacterial pathogens which cause chronic inflammatory disease - one which almost certainly includes multiple species and bacterial forms. but are unable to actually kill the pathogens. For this reasons, patients on the MP take olmesartan, which activates the innate immune response. In this respect, olmesartan is distinct from other ARBs such as telmisartan, which has been shown to be a strong VDR antagonistA substance such as 25-D or certain bacterial ligands which inactivates the Vitamin D Receptor the receptor which transcribes the genes necessary for a proper innate immune response..3) Molecular modeling has revealed that olmesartan binds and activates the Vitamin D Receptor among others. This action is validated by in silicoExperiment technique performed on computer or via computer emulation. data.4)
Because the VDR itself is in constant motion and it is affected by the forces on the VDR from adjacent molecules, olmesartan has an ability to stay in that binding pocket for only a few hours before it is either ejected, or the VDR itself is pulled apart by outside forces.
Olmesartan docks into several different cellular receptors to provide a variety of actions. Thus far, these actions appear to be beneficial.
Other ARBs also bind the same nuclear receptors as olmesartan but fail to activate them at the correct level.
Pharmacodynamics
Like other ligands, olmesartan binds to nuclear receptors above a certain concentration. For this reason MP patients do not use time-release tablets of olmesartan. The affinity for the VDR of olmesartan is low, which explains the need for a higher concentration. Although Benicar closes off the Angiotensin II Receptor (AT2R) at below 20mg/day, MP patients need a much higher dose to properly activate the VDR. Thus the displacement of ligands from the VDR is easier to control by dosage than its blockade of the AT2R.
The pharmacodynamic half life of Benicar is not the same as the pharmacokinetic half-life because there is a disassociation constant involved. That means olmesartan loses its affinity for the VDR more quickly than it decays from the bloodstream. So the effective useful lifetime is 6-8 hours rather than the approximately 13 hours of the plasma half-life.
Incidentally, this discrepancy between olmesartan's effect on the VDR and the A-II receptor is why Marshall Protocol patients are advised to avoid sustained-release versions of Benicar.
The unbound Vitamin D Receptor degrades quickly
Peleg and Nguyen observed that in the absence of an agonist such as 1,25-D, the VDR suffers from polyubiquitination and proteasome-mediated degradation in relatively short course – in the sub-4 hour region, and certainly in the sub-24hour region.5) This study offers some support for why sick patients need more frequent dosing than their healthy counterparts.
Recent published reports on the dynamics of VDR recruitment to promoters of target genes in cultured cells demonstrate that the time course for maximal recruitment of the 1,25D3–VDR complex to the promoter of the CYP24 gene in osteoblasts is 3 h, whereas the time course for maximal recruitment of the VDR to the promoter of this gene in IECs is only 30 min [Kim et al., 2005; Meyer et al., 2006].
S. Peleg and C. V. Nguyen 6)
Infected cells have VDRs with a shorter life span
Some intracellular infections (notably Shigella), upregulate activity of the caspases, which are proteases that cleave the VDR.7) When the VDR is broken apart by the caspases, it is highly likely that any ligands bound to it (such as olmesartan) would stay bound to the fragments of the protein. Therefore, a VDR agonist would be effective over shorter periods of time in patients with infected cells.
Bacterial products down-regulate the expression of the VDR
Lipopolysaccharide (LPS), a part of the bacterial cell wall, reduces VDR protein levels 8)
Pregnane X Receptor (PXR) agonist
Many patients who have begun the MP find that their levels of 1,25-D drop dramatically in the first weeks. This can be explained by pointing to olmesartan's ability to activate both the VDR (discussed previously) and, quite possibly, the Pregnane X Nuclear Receptor (PXR).
Based on [an in silico] model published more recently I would today opine that Benicar is more likely a PXR agonist at this point, as it is active in the same PXR residues as the known agonist SR12813. The gene SR12813 is confirmed to transcribe is that for CYP3A4 [an enzyme which breaks down 1,25-D].
Trevor Marshall, PhD
As discussed further here, 1,25-D tends to be higher than normal in patients with chronic disease and high levels of 1,25-D interfere with the actions of other nuclear receptors – which themselves produce antimicrobial peptidesBody’s naturally produced broad-spectrum antibacterials which target pathogens.. Therefore, a reduction in 1,25-D can contribute to a certain amount of symptom relief.
Olmesartan may also exert its palliative effects through binding other key receptors.
Other beneficial effects
Examples of some of the documented protective effects of ARBs include the ability to:
Cardiovascular disease
Several prospective, randomized studies show vascular benefits with olmesartan medoxomil: reduced progression of coronary atherosclerosis in patients with stable angina pectoris (OLIVUS); decreased vascular inflammatory markers in patients with hypertension and micro- (pre-clinical) inflammation (EUTOPIA); improved common carotid intima-media thickness and plaque volume in patients with diagnosed atherosclerosis (MORE); and resistance vessel remodeling in patients with stage 1 hypertension (VIOS).
R. Preston Mason21)
Olmesartan and other ARBs have been used to block various bad effects of Angiotensin II, including heart failure. In this regard, olmesartan has been shown to:-
- protect the heart from damage from inflammation in myocarditis22)
- ameliorate acute experimental autoimmuneA condition or disease thought to arise from an overactive immune response of the body against substances and tissues normally present in the body myocarditis, in rats, suppressing cytotoxic myocardial injury 23)
- prevent acute left ventricular dysfunction24)
- lower C-reactive protein, one of the acute phase proteins that increase during systemic inflammation25)
- act as an antiarrhythmic26)
- block the production of Angiotensin II, thus improving mortality rates in heart failure patients27)
- This study demonstrated that olmesartan reduced angiotensin II and aldosterone levels more effectively than azilsartan, resulting in a stable antihypertensive effect. Olmesartan also had an inhibitory effect on cardiac hypertrophy. Accordingly, it may be effective for patients with increased RAAS activity after cardiac surgery or patients with severe cardiac hypertrophy. 28)
Pharmacotherapy targeting the renin-angiotensin system [the mechanism of the ARBs] is one of the most effective means of reducing hypertension and cardiovascular morbidity.29) 30)
Nien-Chen Li et al.31)
Olmesartan has also been shown to
Kidney disease
Main article: Benefits of olmesartan for patients with kidney disease
A number of studies have found that olmesartan and other ARBs possess various ways of protecting the kidneys from the effects of inflammation and cytokine damage:
- in circadian rhythms between HR and MAP in CKD: Synchronization between the two rhythms was progressively lost as renal function deteriorated, and Olmesartan partly restored the synchronization 37)
- in hypertensive patients with CKD, olmesartan add-on therapy improves the ambulatory BP profile via a preferential reduction in nighttime BP with concomitant renal injury inhibition 38)
- results suggest olmesartan can help decrease plasma AGE levels in patients on HD 39)
- renal protective effects of olmesartan may be better than those of other ARBs 40)
- olmesartan may uniquely increase urinary ACE2 level, which could offer additional renoprotective effects 41)
Historical note: early insights into the necessity of frequent ARB dosing
In August 2002, Trevor Marshall and Frances (Liz) Marshall published a NetPrint about valsartan (Diovan), in which they reported that the once daily dosing of the ARB caused psychedelic dreams and psychotic events in two sarcoidosis patients. On the theory that these symptoms were caused by changes in plasma concentration, the frequency of the dosing of ARB was increased, which ended up reducing symptoms of disease including psychedelic dreams. This early insight into ARBs anti-inflammatory effects led Marshall to conclude that for an ARB to provide symptomatic relief, it was necessary to use more frequent dosing than typical. Marshall would later go on to recommend frequent dosing of another ARB, olmesartan, as a part of the Marshall Protocol.
Recent studies in preventive effects of Olmesartan in patients
- treatment with olmesartan inhibited bone loss 42)
- olmesartan protects endothelial cells against oxidative stress-mediated cellular injury 43)
- decreases viability of malignant cell lines44)
- carotid IMT and BP decreased similarly with olmesartan and atenolol; but only olmesartan reduced the volume of larger atherosclerotic plaques 45)
- improvement of Plasma Biomarkers after switching stroke patients from other Angiotensin II Type I Receptor Blockers to Olmesartan 46)
- improvement of glycemic control & insulin resistance was only observed in olmesartan group 47)
- OLM substantially delayed the development of left ventricular remodeling in type 2 diabetes 48)
- prevention of microalbuminuria in patients with type 2 diabetes and hypertension 49)
July 2014 data suggest 40 & 80 mg olmesartan are able to significantly remodel & destiffen the arterial wall material during long-term treatment, partly independently of blood pressure, compared with 20 mg. hyper.ahajournals.org/content/early/2014/07/07/HYPERTENSIONAHA.114.03282.reprint 50)
Related publications and presentations
[PMID: 20639756] [PMCID: 2955835] [DOI: 10.1097/MOG.0b013e32833d4b9f]
[PMID: 15936743] [DOI: 10.1016/j.cellimm.2005.04.013]
[PMID: 20222053] [DOI: 10.1002/bdd.699]
[PMID: 16403216] [PMCID: 1360063] [DOI: 10.1186/1742-4682-3-1]
[PMID: 20564192] [DOI: 10.1002/jcb.22606]
[PMID: 17696608] [PMCID: 1941748] [DOI: 10.1371/journal.ppat.0030111]
[PMID: 15876428] [DOI: 10.1016/j.cellimm.2005.03.004]
[PMID: 20068258] [PMCID: 2806632] [DOI: 10.1136/bmj.b5465]
[PMID: 12503978] [DOI: 10.1001/jama.289.1.65]
[PMID: 12871826] [PMCID: 1573934] [DOI: 10.1038/sj.bjp.0705339]
[PMID: 15127887] [DOI: 10.1291/hypres.27.293]
[PMID: 23775504] [DOI: 10.1007/s00210-013-0886-8]
[PMID: 12709417] [DOI: 10.1096/fj.02-0063fje]
[PMID: 17560613] [DOI: 10.1016/j.mvr.2007.05.001]
[PMID: 19303015] [DOI: 10.1053/j.gastro.2009.02.081]
[PMID: 15837532] [DOI: 10.1016/j.regpep.2004.12.015]
[PMID: 21504378] [DOI: 10.3109/08037051.2011.575570]
[PMID: 19763608] [DOI: 10.1007/s00424-009-0725-4]
[PMID: 22283774] [DOI: 10.2174/138161212799436593]
[PMID: 21796255] [PMCID: 3141913] [DOI: 10.2147/VHRM.S20737]
[PMID: 16336207] [DOI: 10.1042/CS20050299]
[PMID: 15879491] [DOI: 10.1152/ajpheart.00078.2005]
[PMID: 15297251] [DOI: 10.1152/ajpheart.00221.2004]
[PMID: 16939632] [DOI: 10.1111/j.1527-3466.2006.00033.x]
[PMID: 16094406] [DOI: 10.1038/sj.jhh.1001933]
[PMID: 16534230] [DOI: 10.1159/000090189]
[PMID: 27086671] [PMCID: 4909997] [DOI: 10.5761/atcs.oa.16-00054]
[PMID: 15531767] [PMCID: 2556374] [DOI: 10.1056/NEJMoa042739]
[PMID: 17984484] [DOI: 10.7326/0003-4819-148-1-200801010-00189]
[PMID: 25275251] [DOI: 10.1097/HJH.0000000000000313]
[PMID: 19124398] [DOI: 10.1177/1753944707085982]
[PMID: 20202514] [DOI: 10.1016/j.jacc.2009.09.062]
[PMID: 19892999] [DOI: 10.1161/STROKEAHA.109.559989]
[PMID: 25001274] [DOI: 10.1161/HYPERTENSIONAHA.114.03282]
[PMID: 23511341] [DOI: 10.1097/HJH.0b013e32836043c9]
[PMID: 23154587] [PMCID: 3594468] [DOI: 10.1038/hr.2012.184]
[PMID: 22149003] [DOI: 10.3109/10641963.2011.628726]
[PMID: 24384547] [PMCID: 3862195] [DOI: 10.1016/j.curtheres.2013.02.002]
[PMID: 24842388] [DOI: 10.1093/ajh/hpu086]
[PMID: 25363367] [DOI: 10.1111/ggi.12406]
[PMID: 25904217] [DOI: 10.1007/s10157-015-1111-5]
[PMID: 28666209] [DOI: 10.1016/j.biopha.2017.06.074]
[PMID: 25891757] [DOI: 10.1016/j.jstrokecerebrovasdis.2015.03.015]
[PMID: 23303198] [DOI: 10.1507/endocrj.ej12-0326]
[PMID: 22418908] [DOI: 10.1097/HJH.0b013e328351856d]
[PMID: 20015057] [DOI: 10.1111/j.1468-3083.2009.03549.x]
[PMID: 16124358]