Related articles: Irritable bowel syndrome, Celiac disease

Table of Contents
Inflammatory bowel disease (Crohn's disease and ulcerative colitis)
Introduction
The metagenomic approach allowed us to detect a reduced complexity of the bacterial phylum Firmicutes as a signature of the faecal microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms. in patients with CD. It also indicated the presence of new bacterial species. 1)
Gut microbial induction of host immune maturation exemplifies host-microbe mutualism. We colonized germ-free (GF) mice with mouse microbiota (MMb) or human microbiotaThe bacterial community in the human body. Many species in the microbiota contribute to the development of chronic disease. (HMb) to determine whether small intestinal immune maturation depends on a coevolved host-specific microbiota. Gut bacterial numbers and phylum abundance were similar in MMb and HMb mice, but bacterial species differed, especially the Firmicutes. HMb mouse intestines had low levels of CD4(+) and CD8(+) T cells, few proliferating T cells, few dendritic cells, and low antimicrobial peptide expression-all characteristics of GF mice. Rat microbiota also failed to fully expand intestinal T cell numbers in mice. Colonizing GF or HMb mice with mouse-segmented filamentous bacteria (SFB) partially restored T cell numbers, suggesting that SFB and other MMb organisms are required for full immune maturation in mice. Importantly, MMb conferred better protection against Salmonella infection than HMb. A host-specific microbiota appears to be critical for a healthy immune system. 2)
Evidence of infectious cause
These papers are really helpful.3) 4)
- Patient response to immunosuppressive medications – Temporary symptomatic remission after taking immunosuppressive medications implies a decrease in the strength of the bacterial die-off reaction known as immunopathologyA temporary increase in disease symptoms experienced by Marshall Protocol patients that results from the release of cytokines and endotoxins as disease-causing bacteria are killed.. That none of these medications make patients better over the long-term is also telling. Several immunosuppressants are used to treat Crohn's including glucocorticoids such as prednisone and budesonide, aminosalicylates such as sulfasalazine and mesalamines, immunomodulators such as azathioprine and methotrexate and biologics such as remicade/infliximab and adalimumab.
- Presence of microbes – Bacteria have long been suspected to be the cause of Crohn's Disease. Detection rates for bacterial DNA from L-formsDifficult-to-culture bacteria that lack a cell wall and are not detectable by traditional culturing processes. Sometimes referred to as cell wall deficient bacteria. (a portion of the metagenomic microbiotaThe community of bacterial pathogens including those in an intracellular and biofilm state which cause chronic disease. that cause disease) in Crohn's tissues is variable due to “the fastidious culture requirements of the cell wall-deficient forms.” 5) The following types and species of bacteria have been found in patients with Crohn's:
De Hertogh et al provide a thorough review of the “unidentified persistent pathogen” theory, stating:
Various environmental factors may play a role in the development of CD [Crohn's Disease], but microbes are most consistently implied. This theory is based on epidemiological, clinicopathological, genetic and experimental evidence. 13)
Other researchers have come to similar conclusions:
M. avium subsp paratuberculosis, adherent-invasive E. coli and Candida are good candidates for an infectious aetiology of Crohn's disease on the basis of genetic susceptibility, which relates to impaired function in the defence against intracellular bacteria.
De Chambrunet et al. 14)
The bacterial community, in whole or in part, resident in the bowel of humans is considered to fuel the chronic immune inflammatory conditions characteristic of Crohn's disease and ulcerative colitis. Chronic or recurrent pouchitis in ulcerative colitis patients is responsive to antibiotic therapy, indicating that bacteria are the etiological agents.
Sokol et al. 15)
Bacterial exotoxins downregulate cathelicidin Family of antimicrobial peptides found primarily in immune cells and transcribed by the Vitamin D Receptor. (hCAP-18/LL-37) and human beta-defensin An antimicrobial peptide found primarily in immune cells and transcribed by the Vitamin D Receptor. 1 (HBD-1) expression in the intestinal epithelial cells. 16)
More evidence
PubMed reviews on the microbiology of inflammatory bowel diseases
58. El Zaatari FA, Osato MS, Graham DY. Etiology of Crohn's disease: the role of Mycobacterium avium paratuberculosis. Trends Mol Med. 2001;7:247-252. 59. Harris JE, Lammerding AM. Crohn's disease and Mycobacterium avium subsp. paratuberculosis: current issues. J Food Prot. 2001;64:2103-2110.
Crohn's disease
Parasitization of vitreous leukocytes by mollicute-like organisms. Am J Clin Pathol. 1989 Mar;91(3):259-64. Johnson LA, Wirostko E, Wirostko WJ. Crohn's disease uveitis
“Uveitis is a symptom, and has many identified causes and associations at this point. Some cases are widely accepted to be due to bacteria or viruses. Other cases are associated with “autoimmune diseases” like Crohns disease and rheumatic arthritis. I suspect that these other cases are due to CWD bacteria. There are several interesting papers that explore this. The researchers found cell-wall deficient bacteria (sometimes called mollicute-like organisms) in the vitreous fluid of patients with sardoidosis, Crohn’s disease, ulcerative colitis, juvenile rheumatoid arthritis, etc.”
Mycobacterium
Aussie-native Dr. Thomas Borody, who was awarded the Marshall Prize in Australia for innovative scientific research and who lectured 3/06 on Long Island, has proposed that Crohn's disease, is caused by Mycobacterium avium paratuberculosis (or MAP for short). The microbe is a distant relative of the tuberculosis and leprosy bacteria.
MP patient reported
Kenc wrote:
This pathogen was suspected in the early part of the last century soon after Crohn's disease was first identified. However, since researchers could not isolate it in Crohn's disease patients they gave up and turned to the concept of Crohn's disease being an immune disorder and so began the big push for immunosuppresive drugs.
Within the last decade there has been a resurgence of interest in MAP. Most Crohn's patients testing positive for MAP (about 45%) have responded well to long term antibiotic therapy in clinical trials (see research by Ira Shafran and by T. Borody). Their response was better than any immunosuppresive drug therapy I know. However, since not all Crohn's patients tested positive for MAP the medical community has remained skeptical of the claim that Crohn's disease is caused by MAP.
I believe the pathogenesis behind the Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. includes the infection by one or more types of bacteria. That would account for the other 55% of Crohn's patients who didn't test positive for MAP. Furthermore the MP should work better than an antibiotic treatment for MAP alone since it would help the immune system to eradicate the other types of bacterium that could be present as well along with MAP.
I tested positive for two other types of bacteria other than MAP. One type of these bacterium comes from tics. I did not take a test for MAP. So, my case is evidence that a Crohn's patient can have non-MAP infections as well.
In spite of the increasing evidence for a microbial cause of Crohn's disease, most (>95% I believe) of the research money available for Crohn's disease, including money from charitable organizations, appears to be directed towards the development of immunosuppressive drugs. The latest craze seems to be for TNF-alpha blockers like Remicade.
The most amazing research I've seen was for MS patients. The idea was to kill all the white blood cells in an MS patient and then use stem cells taken earlier from the patient to repopulate the white blood cells. Of course about half of the patients died. This is modern medical research at its finest! It worked out OK for those that survived. Looking at this with MP eyes, I can see this as a drastic way of getting rid of CWD bacterium in the white blood cells - kill all the cells.
Antibiotics
Researchers at Cedars-Sinai Medical Center have found that a nonabsorbable antibiotic – one that stays in the gut – may by be an effective long-term treatment for irritable bowel syndrome (IBS), a disease affecting more than an estimated 20 percent of Americans. The findings, which showed that participants benefited from the antibiotic use even after the course of treatment ended, support previously published research identifying small intestine bacterial overgrowth (SIBO) as a possible cause of the disease. (Nov. 8, 2005)
“This study is important as it is the first to show that the use of targeted antibiotics results in a more significant and long-lasting improvement in IBS symptoms,” said Mark Pimentel, M.D., director of the GI Motility Program at Cedars-Sinai. “These results clearly show that antibiotics offer a new treatment approach – and a new hope – for people with IBS.”
The randomized, double blind study involved 87 patients. Those on the rifaximin experienced a 37 percent overall improvement of their IBS symptoms as compared to 23 percent on the placebo. Among study subjects whose primary symptom was diarrhea, those on the antibiotic showed more than twice the improvement of those on the placebo (49 percent vs. 23 percent). Patients received the drug (or placebo) for 10 days and were then followed for a total of 10 weeks. Participants kept a stool diary, took a questionnaire and were given methane breath tests. The positive effects of the drug were shown to continue throughout most of the 10-week study, not just during the actual antibiotic course.
Because the cause of IBS has been elusive, treatments for the disease have historically focused on reducing its symptoms – diarrhea and constipation – by giving medications that either slow or speed up the digestive process. In 2000, Pimentel linked bloating, the most common symptom of IBS, to bacterial fermentation, showing that small intestine bacteria overgrowth (SIBO) may be the causative factor in IBS (The American Journal of Gastroenterology, Dec. 2000).
To show evidence of small intestine bacterial overgrowth, participants in both studies were given a lactulose breath test, which monitors the level of hydrogen and methane (the gases emitted by fermented bacteria) on the breath. In the first study, an abnormal breath methane profile was shown to be 100 percent predictive of constipation-predominant IBS. In the current study, the correlation between the amount of methane and the amount of constipation was confirmed, another key finding.
“We were pleased – but not surprised – with the results of this study,” said Pimentel.
Irritable Bowel Syndrome is an intestinal disorder that causes abdominal pain or discomfort, cramping or bloating and diarrhea and constipation. It is a long-term condition that usually begins in adolescence or in early adult life. Episodes may be mild or severe and may be exacerbated by stress. It is one of the top ten most frequently diagnosed conditions among U.S. physicians and affects women more often than men.
Rifaximin is made by Salix Pharmaceuticals, Inc. Funding for the study was provided by Salix Pharmaceuticals, Inc.
One of only five hospitals in California whose nurses have been honored with the prestigious Magnet designation, Cedars-Sinai Medical Center is one of the largest nonprofit academic medical centers in the Western United States. Additional information is available at https://www.cedars-sinai.edu
Targeted Antibiotics Lead to Long-lasting Improvement in IBS Symptoms
It is apparently very rare for a patient to actually be allergic to OlmesartanMedication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor. Also known by the trade name Benicar. (see Research below). An alternative approach where there is present a confirmed mycobacterial infection was recorded here MAP Abx .
Antimicrobial peptides
Explaining how the antimicrobial peptidesBody’s naturally produced broad-spectrum antibacterials which target pathogens. (the body's own antibiotics) are employed against the pathogens which cause Crohn's disease.
The VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response. is responsible for the Cathelicin Anti-Microbial Peptides. It is also responsible for transcribing the genes of the Beta Defensins.
This presentation explains how those, and the other Defensins, are important, and I think that those of you who track the science will find it very interesting indeed.
The presentation runs for 30 minutes. Crohns
Serum levels of 'vitamin' D
BACKGROUND: The active form of vitamin D (1,25(OH)2D3) has been shown to inhibit development of inflammatory bowel disease (IBD) in IL-10 KO mice.
Data point to a critical role for the VDR and 1,25(OH)2D3 in control of innate immunityThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease. and the response of the colon to chemical injury. 17)
Department of Internal Medicine, University of Oulu, Finland. OBJECTIVES. To explore the relationships between vitamin D intake, serum parathyroid hormone (PTH) and 25-hydroxyvitamin D (250HD) concentrations, and bone mineral density (BMD) in inflammatory bowel disease (IBD). SUBJECTS. One hundred and fifty randomly selected patients with IBD from the hospital register and 73 healthy controls.
Patients with IBD have lower serum levels of 250HD than healthy controls, but similar serum PTH concentrations and vitamin D intake. Vitamin D intake, and the serum levels of 250HD and PTH are not associated with BMD, and malabsorption is unlikely to be a major factor in the aetiology of bone loss in unselected IBD patients. 18)
Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum.
The number of E. coli in situ correlated with the severity of ileal disease (rho 0.621, P<0.001) and invasive E. coli was restricted to inflamed mucosa. E. coli strains isolated from the ileum were predominantly novel in phylogeny, displayed pathogen-like behavior in vitroA technique of performing a given procedure in a controlled environment outside of a living organism - usually a laboratory. and harbored chromosomal and episomal elements similar to those described in extraintestinal pathogenic E. coli and pathogenic Enterobacteriaceae. These data establish that dysbiosis of the ileal mucosa-associated flora correlates with an ileal Crohn's disease (ICD) phenotype, and raise the possibility that a selective increase in a novel group of invasive E. coli is involved in the etiopathogenesis to Crohn's disease involving the ileum. 19)
A study suggests that the bacteria-immune system 'fight' continues after the instigator bacteria have been cleared by the body, according to Andrew Gewirtz. The fight can result in metabolic syndrome, an important factor in obesity, or inflammatory bowel disease (IBD).
“The implication at present is that it is very important to control the early environment,” Professor Gewirtz said. “We need to examine how this can be achieved – perhaps via breastfeeding, a more diverse diet, probiotics are possibilities.”
Metabolic syndrome involves risk factors, including obesity, which can lead to cardiovascular disease, diabetes and stroke. According to the American Heart Association, about 35 percent of adults are affected by this syndrome.
IBD, which includes Crohn's disease and ulcerative colitis, happens when the intestines become inflamed, leading to abdominal cramps and pain, diarrhea, weight loss and bleeding.
“It is increasingly apparent that bacteria are playing a role in healthy development, and need to be properly managed by the mucosal immune system to avoid inflammatory diseases” Gewirtz explained. 20)
Patient interviews

Lyme disease, irritable bowel syndrome/ulcerative colitis, radiculitis
Read the interview
Interviews of patients with other diseases are also available.
Research
Findings suggest that neither olmesartan nor other ARBs were associated with diarrhea among patients undergoing endoscopy. The spruelike enteropathy recently associated with olmesartan is likely a rare adverse effect and milder presentations are unlikely. 21)
In a methotrexate-induced model of intestinal mucositis, olmesartan reduced inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue. and induced enteropathy characterized by severe diarrhea, weight loss, and reduced sucrose activity. 22)
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium. 23)
It is now known that epithelial cells have the capacity to secrete and respond to a range of immunological mediators and this suggests that these cells play a prominent role in the pathogenesis of IBD. Current knowledge about the intestinal epithelium has mainly been obtained using models based on animal cells, transformed human intestinal cell lines and isolated cells from resected colonic bowel segments. Species difference, malignant origin and confounders related to surgery, obviously make these cell models however less applicable for patophysiological studies.
Using a functional peptide cleavage assay it was shown that epithelial cells secreted proteolytic active enzymes and that the functional MMP activity was increased in inflamed IBD mucosa.
Our results revealed that colonic epithelial cells express TLR9, a key pattern recognition receptor. Interestingly, the differentiated epithelial cells, which have been exposed to the luminal bacterial flora in vivoA type of scientific study that analyzes an organism in its natural living environment., were unresponsive to TLR9 ligand stimulation, contrasting findings in the epithelial cell line HT-29 that is cultured continuously in bacteria free environment.
'Western'-style diets, high in fat/sugar, low in fibre, decrease beneficial Firmicutes that metabolise dietary plant-derived polysaccharides to SCFAs and increase mucosa-associated Proteobacteria (including enteric pathogens). 24)
Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. microbiology research
Butyric acid in functional constipation 25)
Butyric acid in irritable bowel syndrome 26)
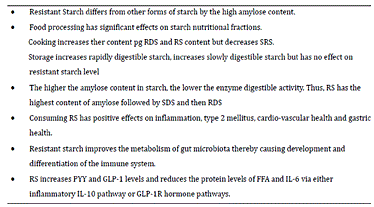
Resistant Starch Regulates Gut Microbiota: Structure, Biochemistry and Cell Signalling
immune mechanisms and interactions between cells of the immune system and tissue environment 27)
[PMID: 1856500] [DOI: 10.1093/infdis/164.2.437]
[PMID: 22726443] [PMCID: 3442780] [DOI: 10.1016/j.cell.2012.04.037]
[PMID: 16849736] [DOI: 10.1099/jmm.0.46498-0]
[PMID: 17699621] [PMCID: 1959459] [DOI: 10.1073/pnas.0706625104]
[PMID: 15074643]
[PMID: 6134652]
[PMID: 7729631] [PMCID: 7127655] [DOI: 10.1016/0016-5085(95)90687-8]
[PMID: 12843021] [PMCID: 165291] [DOI: 10.1128/JCM.41.7.2915-2923.2003]
[PMID: 780185]
[PMID: 18240341] [PMCID: 2687051] [DOI: 10.3748/wjg.14.845]
[PMID: 18622157] [DOI: 10.1097/MOG.0b013e3283023be5]
[PMID: 18275077] [DOI: 10.1002/ibd.20392]
[PMID: 18717821] [DOI: 10.1111/j.1462-5822.2008.01227.x]
[PMID: 17397543] [PMCID: 1852118] [DOI: 10.1186/1471-2172-8-5]
[PMID: 8568480] [DOI: 10.1046/j.1365-2796.1996.420765000.x]
[PMID: 18043660] [DOI: 10.1038/ismej.2007.52]
[PMID: 22863420] [PMCID: 4310462] [DOI: 10.1016/j.chom.2012.07.004]
[PMID: 25023670] [PMCID: 4157109] [DOI: 10.1016/j.mayocp.2014.05.012]
[PMID: 25947920] [DOI: 10.1248/bpb.b14-00847]
[PMID: 25557335]
[PMID: 26011307] [PMCID: 4949558] [DOI: 10.1111/apt.13248]
[PMID: 24868272] [PMCID: 4027827] [DOI: 10.5114/pg.2013.38731]
[PMID: 24868283] [PMCID: 4027835] [DOI: 10.5114/pg.2013.39917]
[PMID: 28126697] [DOI: 10.1016/j.advms.2016.09.001]
[PMID: 14766966] [PMCID: 357038] [DOI: 10.1073/pnas.0307317101]
[PMID: 16702850] [DOI: 10.1097/00042737-200606000-00007]
[PMID: 20203678] [DOI: 10.1038/nrgastro.2010.1]
[PMID: 20224153]
[PMID: 11157137] [DOI: 10.1093/rheumatology/40.1.15]