Related articles: Kidney Capacity and Function, Electrolyte levels, Olmesartan & CKD Protection

Table of Contents
Kidney disease
Therapies
Dialysis
Dialysis is primarily used to provide an artificial replacement for lost kidney function in people with renal failure. The death rate from cardiovascular disease for dialysis patients is much higher than the general population, regardless of age.1) Patients with renal failure are not only exposed to higher volumes of water in their lifetime than the general population, but the barrier between blood and dialysis fluid is generally in the form of a nonselective semipermeable membrane, providing a direct route for any contaminants into the bloodstream. Consequently many of the permitted levels of contaminants in drinking water have the potential to cause problems in dialysis patients.2)
It is known that tap water harbors potentially pathogenic micro-organisms that can pose a significant health threat to patients, especially those people with compromised immune systems and haemodialysis.3)4) For decades, hospital water sources have been known to be reservoirs of nosocomial pathogens, especially organisms of the Pseudomonas sp. yet guidelines for preventing such infections do not exist.5)6)
Today, specialists in infectious diseases are considering point-of-use filters. They presume that the filters achieve a greater than 99% reduction in total heterotrophic plate count of bacteria in the immediate and postflush samples.7)8) They conclude that such filtration units would prevent exposure of high-risk patients to waterborne pathogens. However, the results of Silbaq's 2009 study show that viable ultra-microcells pass through 0.2 micron filtration, and can be cultured on agar, as early as 72 hours after collection. The bacteria were identified using 16S ribosomal DNA sequences. The presence of those ultra-small bacteria in water filtrates would remain undetected by the standard and total heterotrophic plate count.
From the results in this study, one might conclude that the filtration strategies may remove most microbes from water, but cannot prevent it. The ability of UMC to pass through the 0.2 m filters and even 0.1 m filters might select for new emerging pathogens from diverse phyla such as S. maltophilia and Pandoraea sp. which are well recognized as pathogens among cystic fibrosis patients.9)
F.S. Silbaq10)
This work shows that chlorinated tap water harbors opportunistic microbes that can pose some health threat to dialysis patients.
Role of Vitamin D Receptor in in chronic kidney disease
Excess 25(OH)D3 worsens tubulointerstitial injury by modulating macrophage phenotype. 11)
Continuously evidence indicates that deficiencies in vitamin D receptorA nuclear receptor located throughout the body that plays a key role in the innate immune response. activation represents one of key players in adversely affecting cardiovascular health, as well as inducing to secondary hyperparathyroidism in chromic kidney disease patients…. Potentially, selective VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response. activators not only reduce serum parathyroid hormone levels minimizing the risk of hypercalcemia and hyperphosphatemia, but also may improve patient health, reducing the risk of cardiovascular disease.
M. Cozzolino et al.12)
Thessaloniki - Greece, June 2010 13)
Abstract Clinical studies have confirmed that administration of vitamin D receptor (VDR) activators offers a survival benefit in hemodialysis patients and may help in preservation of renal function in predialysis patients. Accumulated clinical and mainly experimental data support that in the context of kidney disease, VDR activators exert their beneficial effect not only due to their action on calcium and phosphorus homeostasis, but also through modulation of the response to injury.
They attenuate systemic and renal inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue., and they affect the tissue repair process, reducing renal fibrosis. This aspect of the functions of VDR activators in kidney disease is reviewed in the present manuscript.
from Torino - Italy: Selective vitamin D receptor activation: effect on renal physiopathology. 14)
Abstract The wide distribution of the vitamin D receptor (VDR) suggests that its activators (VDRAs) are involved in diverse organ functions including the cardiovascular, immune, and reproductive systems. These actions are likely to be independent of PTH and calcium/phosphorus levels. Earlier studies had shown that calcitriol was able to favorably influence experimental nephritis, remnant kidney glomerulosclerosis, and interstitial fibrosis, mediated through inhibition of inflammatory cytokinesAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system..
Recently, VDRAs were shown to inhibit the reninangiotensin system (RAS), acting directly on the renin gene promoter. This action is independent of the systemic RAS blockade. VDRAs also inhibit other important gene promoters including NF-kBA protein that stimulates the release of inflammatory cytokines in response to infection and p65, which are known to foster inflammation and fibrogenesis. These multiple actions result in a decrease in macrophage infiltration, fibroblast activation, and endothelial mesenchymal transition in the kidney.
These findings represent the rationale for the use of VDRAs, in association with RAS blocking agents, to counteract the progression of renal injury characterized by inflammation and neofibrogenesis. However, despite promising preliminary results, the human studies available to date do not allow to draw definitive conclusions on this matter.
Sept. 2010 Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease.15)
Abstract Circulating levels of fibroblast growth factor 23 (FGF23) are elevated in patients with early chronic kidney disease (CKD) and are postulated to cause low blood levels of 1,25-dihydroxyvitamin DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol., as well as normal phosphate levels. In order to provide more direct evidence for the pathophysiological role of FGF23 in the settings of mineral ion homeostasis typically seen in early CKD, we studied rats with progressive CKD treated with anti-FGF23 neutralizing antibody. Without antibody treatment, rats with CKD exhibited high circulating levels of FGF23 and parathyroid hormone, low 1,25-dihydroxyvitamin D, and normal serum phosphate levels, accompanied by increased fractional excretion of phosphate. Antibody treatment, however, lessened fractional excretion of phosphate, thus increasing serum phosphate levels, and normalized serum 1,25-dihydroxyvitamin D by increased 1α-OHase and decreased 24-OHase expressions in the kidney. These antibody-induced changes were followed by increased serum calcium levels, leading to decreased serum parathyroid hormone.
Hence, our study shows that FGF23 normalizes serum phosphate and decreases 1,25-dihydroxyvitamin D levels in early-stage CKD, and suggests a pathological sequence of events for the development of secondary hyperparathyroidism triggered by increased FGF23, followed by a reduction of 1,25-dihydroxyvitamin D and calcium levels, thereby increasing parathyroid hormone secretion.
Subclinical kidney infection
Patients who start the MP are often unaware of the fact that their kidneys or liver are infected until blood work comes back out of range. This is due to the fact that inflammation in these organs tends to be “silent.” Before the MP, the kidneys are inflamed because they are infected with the Th1 pathogens. But the patient is largely unaware of the problem because their immune system, which has been weakened by the pathogens, doesn’t kill enough of the pathogens to cause a rise in bacterial byproducts that would be picked up on a blood test.
Once patients activate the innate immune system with olmesartanMedication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor. Also known by the trade name Benicar. and begin rapidly killing the Th1 pathogensThe community of bacterial pathogens which cause chronic inflammatory disease - one which almost certainly includes multiple species and bacterial forms., the resulting immunopathologyA temporary increase in disease symptoms experienced by Marshall Protocol patients that results from the release of cytokines and endotoxins as disease-causing bacteria are killed. causes a rise in bacterial death, the effects of which are finally high enough to show up on lab tests.
Kidney immunopathology is unavoidable and is necessary for recovery using the Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis.. Thus, people on the MP should not be surprised to learn their kidneys are infected. Patients with Th1 diseaseAny of the chronic inflammatory diseases caused by bacterial pathogens. have a high risk of kidney inflammation, and many doctors are unaware of the problem because they have no idea how sick their Th1 patients really are. Kidneys are also one of the organs that suffer significant fibrosis (collagen deposition and scarring) from the inflammatory disease process.
Calcium-alkali syndrome
Calcium-alkali syndrome due to vitamin D administration and magnesium oxide administration.16)
Got Calcium? Welcome to the Calcium-Alkali Syndrome.
Abstract We recommend changing the name of the milk-alkali syndrome to the calcium-alkali syndrome, because the new terminology better reflects the shifting epidemiology and understanding of this disorder. The calcium-alkali syndrome is now the third most common cause of hospital admission for hypercalcemia, and those at greatest risk are postmenopausal or pregnant women.
The incidence of the calcium-alkali syndrome is growing in large part as a result of the widespread use of over-the-counter calcium and vitamin D supplements. Advertising for treatment or prevention of osteoporosis has long encouraged this use.
Intricate mechanisms mediating the calcium-alkali syndrome depend on interplay among intestine, kidney, and bone. New insights regarding its pathogenesis focus on the key role of calcium-sensing receptors and TRPV5 channels in the modulation of renal calcium excretion.
Restoring extracellular blood volume, increasing GFR and calcium excretion, and discontinuing calcium supplementation provide best treatment. 17)
Evidence of infectious cause
Patients experiences
The renal bloods are so much better and the doctor will no longer blame the Benicar for the abnormal readings. During all of that time I was on the 40mg q6h Benicar, plus the occasional 20mg. The creatinine seems to be going down and the eGFR up, towards the end of the zith cycle.
Pundun, MarshallProtocol.com
My eGFR was retested at 53 up from 45 which my doc informally calls a percentage. Proof that decreased light and more Benicar increase it and therefore that it is IP :)
PatrickBurke (July '08), MarshallProtocol.com
Only benicar every 4 hours during day time and a few times once at 3 am. creatinine down from 138 to 116 (two days ago) (range 60-105). it was comforting to see that an increase in benicar did not lead to an increase in creatinine, as my nephrologist speculated it would.
Inge, MarshallProtocol.com
Just to let you know, that since stopping all abx and reducing the Beni to three times a day, things are much improved- as expected they would be. Kidney function: creatinine -89 (was 103), eGFR = 62 (was 52).
Kas, MarshallProtocol.com
Mino 25mg every two days Benicar 40mg every 3 to 5 hours. One week past kidney “crisis.” New test results are almost normal. Whew.
Somadoc, MarshallProtocol.com
I received some good news during my doctor appointment. My kidney functioning tests were normal. My doctor had taken me off the MP meds back in May due to the increase in my creatinine and BUN. I wasn't sure how my tests results would be right now since I was now at the maximum doses of the MP meds. I was keeping my fingers crossed that the results would be good so my doctor wouldn't take me off the MP meds again. My doctor and I were both surprised to see that both my creatinine and BUN were normal.
Mike9a, MarshallProtocol.com
I've had some blood tests back today and creatinine has gone down to 200. Potassium has gone down to 5.2. Urea has slightly gone down to 22.5. Haemaglobin and lymphocyte count are slightly low. So obviously slowing down the herx has helped with bloods. Bloods for me are a definite way of registering the herx because I'm always herxing and I'm not knowing the difference between tolerable and intolerable. Every since I took Benicar I found I was always herxing without the antibiotics and now I've been off Prednisilone for a year I feel like my immune reactions are very turned on and working stronger than when I started so I have found myself also dropping the antibiotic dose to cope with the herx.
Simonc, MarshallProtocol.com
I will have been off abx for 25 days. My serum creatinine is now back down to 71 umol/L (0.81 mg/dL), with an eGFR of 80 mL/min. These are the same as before starting the MP, so my GP was “relieved” when I spoke to her this morning. I haven't been able to stop smiling all day!
Asilan, MarshallProtocol.com
Finally my creatinine values are down in the normal range (101) (first time since I measured them in April). Hemoglobin is also up (11,5). Will slowly increase mino after having been to a nephrologist next week (only reason for seeing the specialist is to maintain a good relation to my doc). Think it is wise to keep creatinine low when I go see the specialist, so that no stopping of meds or anything will be enforced. Just finished a year on the MP, it has been a lot tougher than expected. And the time it takes seems so long. But hanging in there.
Inge 01/04/08, MarshallProtocol.com
My doc also worries about creatinine, but I never pushed for the 24hour test. What I did instead was to try to reduce herx, under the theory that creatinine was related to herx and both related to dying bacteria. What worked was to reduce the herx. It worked like a champ, my creatinine was normal.
What works to stop the herx seems to depend on the person and on the stage of MP. What works to convince a Doc that the Benicar isn't the problem is even more variable, but somehow you have to do it.
Chris, MarshallProtocol.com
Fortunately my doctor was familiar with the MP, as for the first 2 years, my blood tests always returned marked “kidney failure”.
It was only as my IP reduced in the 3rd and 4th year and vitamin D25 remained undetectable that kidney results turned towards acceptable readings. Sallie
Have been to have my kidney function and BP checked and everything is much improved! Having increased my Benicar frequency to 6-hourly (and I've reduced my abx except mino to give my body a rest) my blood pressure has risen to a respectable 94/57. This is remarkable, as it had stabilized at 80/40 since I was in Phase 1. Creatinine, Urea, Urate and eGFR all returning to bearable levels. Only my anaemia remains but I will hope for that to improve slowly.
So this is a lesson to others whose doctors panic and say “Whoa! Your BP is dropping, you should take less of that Benicar.” The counter-intuitive answer is to TAKE MORE instead.
Claudia, MarshallProtocol.com
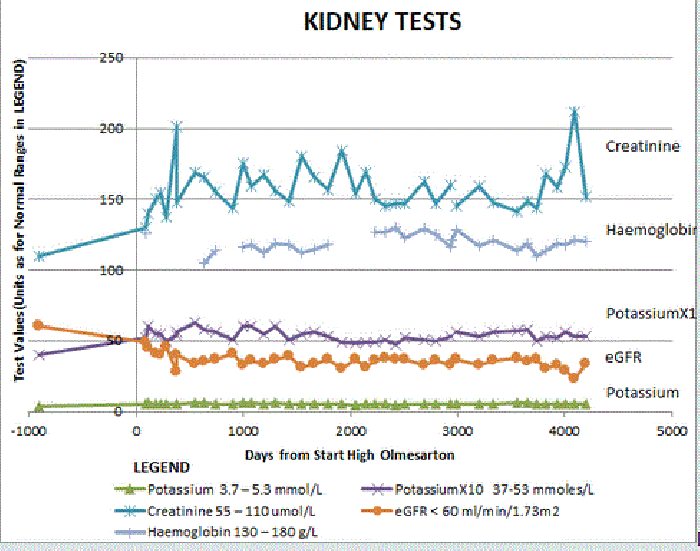
Patient's chart
with notes re eating meat before a test
Creatinine, at 130 umole/L, was above normal range from the start. It took a few months to reach around 150 umole/L which seemed to become the basic level for the the next 11 years with peaks above that level being associated with other factors. The two anomalous peaks above 200umole/L were associated with having meat meals too close to the time of the test. The creatine and creatinine content in vertebrate muscle, or creatine supplements, can be absorbed to add to the body's potential to generate serum creatinine (70Effect of a Cooked Meat Meal on Serum Creatinine and Estimated Glomerular Filtration Rate in Diabetes-Related Kidney Disease | Diabetes Care). After the early 200+ peak, from a sample taken after a light chicken lunch, meat was avoided for a day or two before sampling for subsequent tests.
The other exception was the second last one, for which meat was eaten the night before sampling. Apart from the aberrations arising from meat eating, eGFR values remained in the 30 to 40 range throughout the 11 year period. Thus the MP treatment, while inducing an initial fall in eGFR, has subsequently allowed the kidney function to remain stable.
[PMID: 20411052] [PMCID: 2843577]
[PMID: 12953025] [DOI: 10.1093/ndt/gfg1074]
[PMID: 9950177] [DOI: 10.1046/j.1525-1594.1999.06275.x]
[PMID: 16329897] [DOI: 10.1016/j.femsec.2004.10.015]
[PMID: 12090885] [DOI: 10.1001/archinte.162.13.1483]
[PMID: 12861087] [DOI: 10.1097/00001432-200308000-00006]
[PMID: 15940113] [DOI: 10.1016/j.ajic.2005.03.012]
[PMID: 15940115] [DOI: 10.1016/j.ajic.2005.03.006]
[PMID: 15865277] [DOI: 10.1086/502558]
[PMID: 19040704] [DOI: 10.1111/j.1365-2672.2008.03981.x]
[PMID: 26176830] [DOI: 10.1038/ki.2015.210]
[PMID: 20540037]
[PMID: 19941275]
[PMID: 20844473] [DOI: 10.1038/ki.2010.313]
[PMID: 19185403] [DOI: 10.1053/j.ajkd.2008.11.015]
[PMID: 20413609] [DOI: 10.1681/ASN.2010030255]