Related articles: Test: 1,25-D, Metabolism of vitamin D and the Vitamin D Receptor

Table of Contents
Test: 25-hydroxyvitamin D (25-D)
Also known as 25-hydroxyvitamin D, 25-DThe vitamin D metabolite widely (and erroneously) considered best indicator of vitamin D "deficiency." Inactivates the Vitamin D Nuclear Receptor. Produced by hydroxylation of vitamin D3 in the liver. is the inactive form of vitamin D. Like 1,25-DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol., 25-D has an affinity for the Vitamin D ReceptorA nuclear receptor located throughout the body that plays a key role in the innate immune response. (VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response.), but unlike 1,25-D, it inactivates the Receptor.
When researchers or journalists talk about vitamin D deficiency, they are invariably talking about low levels of 25-D as opposed to 1,25-D. According to the Marshall PathogenesisA description for how chronic inflammatory diseases originate and develop., the body purposefully downregulates levels of 25-D so as to upregulate activity of the VDR.
As the vitamin D metabolite calculator states, any 25-D of 20 ng/ml or higher is immunosuppressive and should be countered by restricting consumption of vitamin D.
If a patient's 25-D is low enough, they typically can enjoy an infrequent splurge of food containing vitamin D.
When to test 25-D
Patients should ask their physicians to order a baseline 25-D test prior to beginning olmesartan (Benicar)Medication taken regularly by patients on the Marshall Protocol for its ability to activate the Vitamin D Receptor.. Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. patients whose 25-D is above 20 ng/ml should continue to be tested every three months. This allows the doctor and the patient to anticipate a possible increase in immunopathologyA temporary increase in disease symptoms experienced by Marshall Protocol patients that results from the release of cytokines and endotoxins as disease-causing bacteria are killed. (IP), which corresponds to a 25-D level of 20 ng/ml.
Intermittent testing (once every six months or longer) can continue thereafter to verify a patient is continuing to successfully avoid food containing vitamin D.
How to test 25-D
MP patients getting their 25-D levels tested should ensure the relevant instructions are closely followed.
Interpreting a 25-D test
Contextual interpretation of a patient's 25-D and 1,25-D results are available using the vitamin D metabolite calculator.
A patient may get a test result, breaking down their 25-D into two different kinds:
- 25 D3 – synthesized on human skin when exposed to light; enters the body when a person consumes animal products that contain vitamin D
- 25 D2 – enters the body when a person consumes plants or fungi that contain vitamin D
Units of measurement
On the Marshall Protocol study site, 25-D levels are typically discussed using ng/ml units rather than pmol/L. The ratio between the two units is: 1 ng/ml = 2.5 nmol/L. To convert nmol/L into ng/ml, multiply by 0.40, as you see in this example:
60 nmol/L * 0.40 = 24 ng/mL
This is also done automatically with the vitamin D metabolite calculator.
Diseases with a low 25-D
Related article: Diseases associated with low levels of 25-D
Lower than normal levels of 25-D have been independently associated both with all-cause mortality1) and dozens of chronic inflammatory diseases ranging from alcoholism 2) to allergies3) to prostate cancer.4)
For this reason, low levels of 25-D can be used (in countries that supplement) as a proxy for chronic disease.
Not a simple fat-soluble vitamin
A recent analysis concluded that surrogate markers for vitamin D exposure including age, vitamin D intake, supplement use, latitude, etc. taken together, could explain only 21 percent of the variation in vitamin D levels between people.5)
As a 2010 Tasmanian study demonstrated, body fat is not simply a passive reservoir for 25-D. The study showed that the associations between body adiposity (fat) measures and change in 25-D completely disappeared after adjustment for leptin (an appetite hormone), diminished after adjustment for IL-6 (a cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system.), but remained unchanged after adjustment for total cholesterol/HDL ratio. Therefore, in addition to season and sun exposure, 25-D levels appear to be determined by metabolic and, to a lesser extent, inflammatory factors, and these appear to mediate the effects of adiposity (body fat) on change in 25-D.6)
Reference range for 25-D
The question of what should be the appropriate reference range for vitamin D involves several issues.
Healthy people who do not supplement have naturally low levels of 25-D
Observational studies show that populations which avoid vitamin D consumption have naturally low levels of 25-D and remain healthy with such levels.
- A study which tested the level of 25-D in 90 “healthy, ambulatory Chilean women” showed that 27% of the premenopausal and 60% of the postmenopausal women had 25-D levels under 20 ng/ml. 7)
- A study on healthy Bangladeshi women found that approximately 80% of the women had a level of 25-D under 16 ng/ml. 8)
- In a 1992 study, healthy full-term infants from China had serum concentrations of 25-D ranging from an average of 5 ng/ml to 14 ng/ml. 9)
Patients with chronic diseases naturally downregulate levels of 25-D
Related article: Mechanisms by which bacteria affect levels of vitamin D
There are several molecular pathways activate in chronic inflammatory disease, which cause levels of 25-D to fall to “deficient” levels. (For example, Reid et al. showed that blood levels 25-D decrease after an inflammatory insult such elective knee arthroplasty.10)) It is in the interest of such patients to have low levels of 25-D, as low levels increase the activity of the VDR – a receptor which, when activated, plays a key role in innate immune function.
Under such circumstances, a patient who supplements with vitamin D may see a rise in 25-D. However, the increase in serum levels of 25-D would not be quite as high as it otherwise would be in a healthy person.
In other words, the body's enzymatic regulation of the D metabolites can be forcefully overridden by heavy dietary and supplemental intake of D precursors.
The explanation presented here for why 25-D is low in patients with chronic disease runs counter to the more commonly given but incorrect description, namely that patients “use up” vitamin D as they would a true vitamin.
Establishing a reference range
The high rate of chronic disease and the presence of vitamin D supplementation has led to a misunderstanding about what constitutes a healthy or normal range for vitamin D. Laboratories establish a “normal” range for the D metabolites results based on studies purportedly looking at the average of all the “healthy” persons who are tested. Clinicians have yet to recognize the reason for low levels of 25-D and usually recommend supplementation with vitamin D. Therefore, lab ranges for 25-D may be skewed higher and higher by the increasingly prevalent use of dietary supplementation.
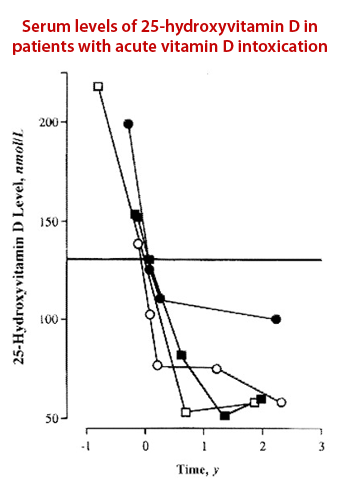
The therapeutic range for Marshall Protocol patients is 11 ng/ml or lower. MP patients often have a 25-D below the detectable limit of 5-7 ng/ml.
Expected rate of decline in 25-D
The rate at which 25-D declines in Marshall Protocol patients tends to vary. Adams et al. (right) showed that the rate of at which 25-D declined among people who taken high amounts of vitamin D supplements and subsequently abstained from supplements is approximately 10.7 ± 3.0 nmol/L per month.11)
Recalcitrant 25-D – why 25-D could remain high over the course of several years
Sometimes, a patient will be on the MP for 18 months or more, and their 25-D will still be elevated above the therapeutic range – that is, greater than 20 ng/ml. Sometimes a patient has been mistakenly consuming food or supplements containing vitamin D. This explanation must never be overlooked.
The best way to check if a patient is taking vitamin D is to carefully review the possible sources of vitamin D including supplements. Many foods and supplements contain unlisted amounts of vitamin D. Patients can try varying their diet and re-testing themselves to see if this changes their measurable level of 25-D.
However, even patients avoiding supplemental vitamin D and without significant body fat – where vitamin D is supposedly stored – can have high levels of 25-D.
Why did her 25-D take so long to drop, especially when she has so little body-fat for “storage”, while obese individual's 25-D can drop quickly? I would postulate that it is because 25-D is not stored, it is metabolized, and the metabolism is out-of-whack.
Trevor Marshall, PhD
Factors that may contribute to unexpected 25-D
Here are several factors may play a role in contributing to unexpected levels of 25-D:
- Yeast may be able to produce a 25-D precursor – Patients who have large yeast overgrowth such as Candida may have elevated levels of 25-D as Candida produces ergosterol, a Vitamin D2Form of vitamin D created by plants and fungi. When ingested the secosteroid is (sometimes) converted into 25-D. Also known as ergocholecalciferol. precursor.12) 13) If this is the case, one would expect a large D2 contribution to total 25-D. This could be assessed if the test is broken down in this fashion, which it not always is.
I have found elevated levels of 25-D in compliant patients. It is usually due to an intestinal Candida overgrowth or biofilm A structured community of microorganisms encapsulated within a self-developed protective matrix and living together.. I have found treating with Nystatin, a non-absorbable antifungal azole effective in reducing Candida load and with resultant marked reduction in 25-D levels, often within 1 to 2 months.
Greg Blaney, M.D.
- Minocycline could be activating other receptors – Another explanation is that the MP itself, through activation of the Pregnane X Nuclear ReceptorIntracellular receptor proteins that bind to hydrophobic signal molecules (such as steroid and thyroid hormones) or intracellular metabolites and are thus activated to bind to specific DNA sequences which affects transcription. (PXR) is contributing to higher than normal levels of vitamin D. Marshall Protocol patients take minocycline, which appears to bind the PXR. A similar action may be generated by olmesartan (Benicar). When active, the PXR transcribes the enzyme CYP3A4, which breaks down 1,25-D. When 1,25-D is broken down, 25-D levels escalate. An activated PXR could explain why some MP patients have stubbornly high levels of 25-D even while being extremely careful to avoid consumption of vitamin D.
- Serum levels of 25-D may not be accurate reflection of cellular levels
As I have indicated in the past I have seen marked changes in vitamin D serum metabolite measurements including 25-D levels that had nothing to do with diet or light exposure. I have also witnessed immunopathology is patients with serum 25-D levels as high as 30 ng/ml. So obviously what is happening intracellularly does not always reflect in extracellular markers.
Greg Blaney, M.D.
25-D after recovery
Endogenous 25-D production will not start to rise until the bacteria have been largely eliminated, which will be in the final few years of MP immunopathology. A healthy 25-D level varies widely, with 25-D being manufactured as the body needs it, and with the maximum level in a healthy person being about 18ng/ml.
Trevor Marshall, PhD
Patients experiences
My feeling worse corresponded to dropping levels of 25-D. Although our “therapeutic range” is <12ng/ml, my own experience of going from 12ish to 4ng fairly rapidly was feeling considerably worse pretty quickly. My own apparent deterioration seems to have begun to reverse over the subsequent 8-9 months. Everyone's different, but perhaps you'll also find this to be the case. So hang in there, and in the meantime do what you can to keep things tolerable.
pgeek, MarshallProtocol.com
Read more
[PMID: 18695076] [PMCID: 2677029] [DOI: 10.1001/archinte.168.15.1629]
[PMID: 11005548] [DOI: 10.1007/s007020070063]
[PMID: 19154546] [DOI: 10.1111/j.1398-9995.2008.01865.x]
[PMID: 15663995] [DOI: 10.1016/j.jsbmb.2004.10.007]
[PMID: 20219959] [PMCID: 2854906] [DOI: 10.3945/ajcn.2009.28908]
[PMID: 20804516] [DOI: 10.1111/j.1365-2796.2010.02267.x]
[PMID: 17290161] [DOI: 10.1097/GME.0b013e31802c54c0]
[PMID: 16500882]
[PMID: 1578308] [DOI: 10.1016/s0022-3476(05)80236-7]
[PMID: 21411617] [DOI: 10.3945/ajcn.110.008490]
[PMID: 9245225] [DOI: 10.7326/0003-4819-127-3-199708010-00004]
[PMID: 6016607]
[PMID: 8561481] [DOI: 10.1146/annurev.mi.49.100195.000523]

