Related article: Summary of research on aging and Olmesartan

This is an old revision of the document!
Table of Contents
Aging (senescence)
The biological basis of aging (also known as senescence) is the subject of debate. A number of theories suggest aging is inevitable, the product of “wear and tear” or a result of some evolutionary necessity. For example, Skulachev argues that aging performs a specific biological function, with the turnover in new members of a species promoting greater evolutionary fitness.1) One of the longstanding problems of such theories is that they fail to account for dramatically different lifespans, or for the fact that chronic diseases are identical in presentation to diseases of the aging. We have a lot to learn about 'diseases of the aging'.
Recovery from some of the diseases of aging
InflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue., But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians. 2)
Health of the hypothalamus may be a controlling factor as demonstrated in a mouse study by Yalin Zhang et al
Mechanistically, hypothalamic stem/progenitor cells contributed greatly to exosomal microRNAs (miRNAs) in the cerebrospinal fluid, and these exosomal miRNAs declined during ageing, whereas central treatment with healthy hypothalamic stem/progenitor cell-secreted exosomes led to the slowing of ageing. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs
and another with senior author Dongsheng Cai, M.D., Ph.D., (professor of molecular pharmacology at Einstein.)
The hypothalamus was known to regulate important processes including growth, development, reproduction and metabolism. In a 2013 Nature paper, Einstein researchers made the surprising finding that the hypothalamus also regulates aging throughout the body. Now, the scientists have pinpointed the cells in the hypothalamus that control aging: a tiny population of adult neural stem cells, which were known to be responsible for forming new brain neurons.
“Our research shows that the number of hypothalamic neural stem cells naturally declines over the life of the animal, and this decline accelerates aging,” says senior author Dongsheng Cai, M.D., Ph.D., (professor of molecular pharmacology at Einstein. “But we also found that the effects of this loss are not irreversible. By replenishing these stem cells or the molecules they produce, it's possible to slow and even reverse various aspects of aging throughout the body.” natural decline in neural stem cells over the life of the animal
According to the Marshall PathogenesisA description for how chronic inflammatory diseases originate and develop., chronic microbes drive the aging process, in light of the fact that diseases and symptoms of aging are at their essence, inflammatory conditions. It is the damage done by microbes coupled with the body's response to these pathogens which is responsible for aging skin, wrinkles, and the wear and tear on organs.
Conventional explanations for aging
A number of unsatisfactory theories have been touted to explain why aging occurs:
- Wear-and-tear theory – According to the wear-and-tear theory of aging, senescence is the result of chance. The human body is constantly wearing out and being repaired. Like components of an aging car, parts of the body wear out from repeated use.
- Aging-clock theory – Aging is programmed into our bodies like a body ticking away from the moment of conception. For example, female mammals are born with a finite number of egg cells in the ovaries that produce estrogen.
- Cross-linkage theory – Connective tissue in the body, such as the skin or the lens of the eye, loses elasticity with advancing age, allowing for the accumulation of harmful molecule.
- Free radicals – Free radicals are highly reactive and toxic when they come into contact with cell structures, thus generating biologically abnormal molecules. The articles pH Balancing and antioxidant supplementation address this theory.
- Cellular theory – According to this theory, the normal body has a finite potential to replicate and maintain functional capacity (Hayflick limit).
Breaking up defunct cells
Article in Press Senolytics in idiopathic pulmonary fibrosis
Selectively ablating senescent cells using dasatinib plus quercetin (DQ) alleviates IPF-related dysfunction in bleomycin-administered mice.
Our first-in-humans open-label pilot supports study feasibility and provides initial evidence that senolytics may alleviate physical dysfunction in IPF 3).
Diseases of the aging are chronic diseases
Moody writes, “Normal aging can be defined as an underlying time-dependent biological process that, although not itself a disease, involves functional loss and susceptibility to disease and death.4) Is aging inevitable?
There are two key problems with most conventional theories of aging. There is simply too much variability in symptoms of aging. The dramatic discrepancy lifespans suggests it's more than just chance which causes the symptoms of senescence.
For example, consider an 18-year old male who has constipation, brain fogThe loss of intellectual functions such as reasoning; memory loss; and other neurological abilities that is severe enough to interfere with daily functioning., and insomnia. Omit his age, and on paper, he sounds like an 80-year old. How are his symptoms qualitatively any different than his 80-year old counterpart's?
According to the Marshall Pathogenesis, diseases of the aging are simply late onset chronic diseases caused by infection.
Aging is a super-category. We’ve gradually lumped together more and more symptoms under the category of natural aging. Many of these symptoms are the same as those caused by diseases that surely have an infectious cause. In that sense, you could view much of what we now call aging as an incapacitating illness that leads to a decrease in function. We know that inflammation and the interaction of the immune system with pathogens can destroy tissue. So it’s not surprising that the tissues of a person who harbors a lot of pathogens would age earlier and alter their biological structure earlier in life. I do believe it is inevitable that people will eventually die of old age, but I suspect that this should generally happen when they are 80-100 years old. But we are increasingly seeing signs of aging-related diseases in people who are much younger.
Paul Ewald, Bacteriality interview
Infection and decline in immune function
A typical feature of aging is a chronic, low-grade inflammation characterized by a general increase in the production of pro-inflammatory cytokinesAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. and inflammatory markers.5) There is even a term for it: inflammaging. According to Franchesci: “A large part of the aging phenotype, including immunosenescence, is explained by an imbalance between inflammatory and anti-inflammatory networks, which results in the low grade chronic pro-inflammatory status we proposed to call inflammaging.”6)
Aging deeply affects (or is affected by!) the human microbiotaThe bacterial community in the human body. Many species in the microbiota contribute to the development of chronic disease.'s homeostasis with the host's immune system:7) 8)
- autoimmuneA condition or disease thought to arise from an overactive immune response of the body against substances and tissues normally present in the body – As people age, their risk for developing an “autoimmune” condition also increases.9) The article on autoimmune conditions discusses why so-called “autoantibodies” are merely antibodies generated in response to pathogenic bacterial cells that have been destroyed as a result of an active immune response.
- macrophage function – Macrophages, which act as “pathogen sensors”, lose the ability to initiate an inflammatory response as people age.10) Microbes use a variety of methods to infect macrophages. For example, Neisseria meningitidis prevents macrophage apoptosis via genes encoding nitric oxide detoxification and a porin, PorB.11)
This theory is based on the fact that genes affecting host organism longevity are represented by subpopulations: genes of host eukaryotic cells, commensal microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms., and non-living genetic elements. ……….. we propose that lifespan and aging are defined by the accumulation of alterations over all genes of macroorganism and microbiome and the non-living genetic elements associated with them. 12)
Could the chronic inflammation associated with aging be caused by pathogens? Given the crudeness of tools now used to measure microbes and the ubiquity of the human microbiota, this seems like a reasonable if not inevitable conclusion as the early studies have begun to suggest.
At present, a study on gut microbiota composition shows that three main modifications occur in faecal microbiota from old frail subjects: a 26-fold reduction in the number of lactobacilli (which stimulate immune functions and help the nutrient absorption), a 3-fold reduction in the number of bacteriodes (which digest polysaccarides, some species are opportunist pathogens) and a 7-fold increase in the number of enterobacteriacee (potentially pathogens)13). Differences in faecal microbiota were also found in a study on people of different countries and ages, including aged and long-lived people14).
E. Cevenini15)
Infections may drive age-related memory loss
- cognitive and functional decline often follow severe sepsis – The researchers focused their attention on 516 individuals who survived hospitalization for severe sepsis and 4517 who survived a nonsepsis hospitalization. The average age of survivors at hospitalization was 76.9 years. Following severe sepsis hospitalization, but not nonsepsis general hospitalization, there was a significant increase in the odds of developing both cognitive and physical dysfunction that persisted throughout the 8-year follow-up period, the researchers report.16)
- aging immune cells in animal models – The decline in brain function associated with disease and old age could be due to the decline in the function of immune cells, which is likely caused by infection. As described in New Scientist, prompted by studies suggesting immune responses can help repair the nervous system, Jonathan Kipnis and colleagues at the University of Virginia created mice that lack CD4 cells, a kind of T-cell. They found the mice performed extremely poorly in tasks involving learning and memory, but when they were injected with CD4 cells from healthy mice, their memories improved.17) Similarly, when he killed CD4 cells in healthy mice, their memory declined. Further animal studies by Kipnis and others show that learning new tasks triggers a mild stress response within the brain, which prompts CD4 cells to rally to the meninges, the membranes that surround the brain. Here, they release IL-4, which both switches off the stress response and tells brain cells called astrocytes to release brain-derived neurotrophic factor, a protein that enhances learning.18) Whether these animal studies are relevant to human learning and memory remains unclear, but there is some indirect evidence to support it. For example, many chemotherapy drugs suppress the immune system, which might explain why some people with cancer develop “chemobrain” - a term used to describe the cognitive problems and memory loss associated with chemotherapy. Sluggish immune cells might also explain why our brains slow down as we age. “The number one cell affected by ageing is the T-cell,” says Kipnis. “I'm not saying it's the only factor leading to age-related dementia, but it could definitely be one of them.”
Research into effects of Olmesartan
Role of vitamin D metabolism
The VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response. plays a plays a crucial, often under-appreciated, role in the innate immune responseThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease.. Further underscoring the potential role of microbes in chronic disease, there are several demonstrated connections between VDR dysfunction and premature aging.
- VDR knockout mice show signs of premature aging – VDR knockout mice (mice that have their Vitamin D Receptors removed) show signs of premature aging:
Overall, VDR KO mice showed several aging related phenotypes, including poorer survival, early alopecia, thickened skin, enlarged sebaceous glands and development of epidermal cysts…. Unlike the wildtype controls, VDR KO mice lose their ability to swim after 6 months of age. Expression of all the genes was lower in old VDR KO mice, but only NF-kappaBA protein that stimulates the release of inflammatory cytokines in response to infection, Fgf-23, p53 and IGF1R were significantly lower. Since the phenotype of aged VDR knockout mice is similar to mouse models with hypervitaminosis D(3), our study suggests that VDR genetic ablation promotes premature aging in mice, and that vitamin D(3) homeostasis regulates physiological aging.
T. Keisala et al.19)
- Vitamin D restriction reduces signs of aging in certain mice – The Klotho-insufficient (klotho) mouse exhibits a syndrome that resembles human aging including retarded growth, osteoporosis, atherosclerosis, ectopic calcification, immunological deficiency, skin and general organ atrophy, hypogonadism and short lifespan.20) This syndrome is mitigated – in one researcher's words, “rescued” – when mice have their vitamin D restricted.21) Interestingly, while murine research is a mainstay of the community of researchers and patients interested in longevity, this line of research has not led for calls to restrict vitamin D in the name of health.
Falls and Fractures
New Prevention Guidelines for Falls and Fractures
The major change is the downgrade of the vitamin D supplementation recommendation for preventing falls from being a B grade (recommended based on high certainty of moderate benefit or on moderate certainty of moderate to substantial benefit) to a D grade (recommended against based on moderate or high certainty of no benefit or that harms outweigh the benefit). For falls prevention, exercise remains a B grade recommendation, and multifactorial interventions remains a C grade (selectively offer) recommendation. Both falls prevention recommendations are consistent with a 2017 meta-analysis of falls prevention interventions.
Recommendations for fracture prevention have not changed, including insufficient evidence to make recommendations on calcium and vitamin D to prevent fractures in men and premenopausal and postmenopausal women.
Diet
Unlike current food pyramid recommendations, vigorous health in maturity may require far more daily fruit and vegetables; less carbohydrate, much less meat and no processed meat.
see Food and drink
Gray hair
Main article: Gray hair
Recent research
Cell membranes are primarily made up of two types of lipids — phospholipids and glycolipids. Inside cells, these lipids bind to a molecule called CD1d that transports them to the surface. Once there, phospholipids stimulate phospholipid-reactive T cells, and glycolipids stimulate a different type of T cell called iNKTs.
On their way to the cell’s surface, phospholipids more easily attach themselves to CD1d molecules, making it more difficult for glycolipids to attach to CD1d. Because of this, it is harder for glycolipids to make it to the surface of the cell. This means that iNKTs cannot be as easily stimulated by glycolipids.
Scientists believe iNKT cells are necessary because they appear to protect cells against the progression of certain cancers and autoimmune diseases. However, iNKT cells are extremely active and can cause alcoholic hepatitis or other types of liver diseases if they are overstimulated. The phospholipid’s ability to more easily bind to CD1d molecules than glycolipids keeps a balance between the two cell types and maintains homeostasis in the immune system. 22)
Read more
- Declining testosterone levels in men not part of normal aging, study finds – A 2012 study found that declining testosterone levels are not an inevitable part of the aging process, as many people think. Men who had declines in testosterone were more likely to be those who became obese, had stopped smoking or were depressed at either clinic visit.
Notes and comments
Lee ” I am 70 now (not 65) when we started this conversation …lolDr's were amazed that I was in such good shape and all fractures were closed and healed well. I would like to say I may not bicycle ride now ..but it's a family thing and I probably will “
(({{pubmed>long:000}}))
<DiseaseHierarchy>
APMIS. 2012 Oct;120(10):773-7. doi: 10.1111/j.1600-0463.2012.02899.x. Epub 2012 Mar 23. Age-related prevalence of Methanomassiliicoccus luminyensis in the human gut microbiome.
Dridi B, Henry M, Richet H, Raoult D, Drancourt M. Source
Unité de Recherche sur les Maladies Infectieuses et Tropicales Emergentes, IHU Méditerranée Infection, Aix-Marseille- Université, Marseille, France.
Abstract
A 16S rDNA sequence-based investigation of methanogenic Archaea in the human stools found Methanobrevibacter smithii in 99.2% and Methanosphaera stadtmanae in 32.6%. The recently described Methanomassiliicoccus luminyensis found by others to be representative of a new order of methanogenic Archaea was found in 4% of stool specimens. The prevalence of M. luminyensis significantly increased with age, contrary to M. smithii and M. stadtmanae.
© 2012 The Authors APMIS © 2012 APMIS.
PMID: 22958284
Inflammatory and Immunological parameters in adults with Down syndrome Maria BF Trotta1*, João B Serro Azul1, Mauricio Wajngarten1, Simone G Fonseca1, Anna C Goldberg2 and Jorge E Kalil1 RESEARCH Open Access Abstract Background: The increase in life expectancy within the general population has resulted in an increasing number of elderly adults, including patients with Down syndrome (DS), with a current life expectancy of about 50 years. We evaluate the parameters of humoral and cellular immune response, the quantitative expression of the regulator of calcineurin1 gene (RCAN1) and the production of cytokines. The study group consisted of adults DS (n = 24) and a control group with intellectual disability without Down syndrome (ID) (n = 21) and living in a similar environmental background. It was evaluated serology, immunophenotyping, the quantitative gene expression of RCAN1 and the production of cytokines. Results: In the DS group, the results showed an increase in NK cells, CD8, decreased CD19 (p < 0.05) and an increase spontaneous production of IFNgamma, TNFalpha and IL-10 (p < 0.05). There was not any difference in RCAN1 gene expression between the groups. Conclusions: These data suggest a similar humoral response in the two groups. The immunophenotyping suggests sign of premature aging of the immune system and the cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. production show a proinflammatory profile.
21496308
As far as I can see, Th1 pathogensThe community of bacterial pathogens which cause chronic inflammatory disease - one which almost certainly includes multiple species and bacterial forms. start to dictate the 'health' of just about everyone as they age. If you draw a graph of 25-DThe vitamin D metabolite widely (and erroneously) considered best indicator of vitamin D "deficiency." Inactivates the Vitamin D Nuclear Receptor. Produced by hydroxylation of vitamin D3 in the liver. levels vs age, they drop steadily after age 40. Something is happening, even during 'healthy aging', that we really ought to understand a little more :)
There is a branch of medicine which is starting to look at Immunity and Aging. Here is a short letter I recently wrote to the editor of one of the journals: http://www.immunityageing.com/content/3/1/12/comments
Trevor Marshall, PhD
Aging, the accumulation of changes in an organism or object over time,
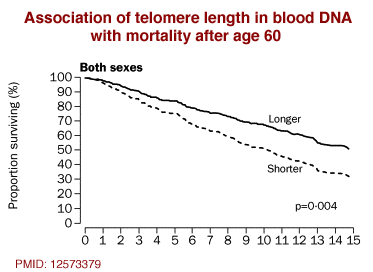
Cawthorn et al examined the telomeres of 143 people over the age of 60.23) Telomeres are DNA sequences on the ends of chromosomes that are gradually lost as cells replicate. The team found that those with shorter telomeres in blood DNA had significantly poorer survival, attributable in part to a 3.18-fold higher mortality rate from heart disease and, tellingly, a 8.54-fold higher mortality rate from infectious disease.
A number of the sickest patients on the Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis., who are killing intracellular bacteria at the fastest rate possible, take over three years to completely recover their health. This hints at the large amount of pathogen-altered DNA that many people, even those who are not yet displaying the hallmarks of Th1 diseaseAny of the chronic inflammatory diseases caused by bacterial pathogens., are carrying.
Study shows that most older adults have signs of brain damage http://bacteriality.com/2008/01/04/brain/
People who haven’t completed the MP consider it inevitable that as they age, they will need to stock up on any number of medications including thyroid medications, cardiac meds, statins, NSAIDS, B/P meds, etc. But people who have reached the later stages of the MP see no reason why they cannot take their level of healing to a maximum – to a point where they will never need these medications and may experience great health during their elder years.
Amy Proal, Top 14 misconceptions about the MP: addressed and explained
EMBO Mol Med. 2010 Jul;2(7):247-57. Angiotensin II revisited: new roles in inflammation, immunology and aging. Benigni A, Cassis P, Remuzzi G. Mario Negri Institute for Pharmacological Research, Bergamo, Italy. Abstract That the renin-angiotensin system (RAS) is involved in regulation of blood pressure, vasoconstriction, sodium intake and potassium excretion is well established. Studies in the last few years have however documented new roles for this molecule as a pro-inflammatory molecule and more recently as a possible pro-fibrotic agent that contributes to progressive deterioration of organ function in disease. Binding of Ang II to its receptors (in particular AT(1)) mediates intracellular free radical generation that contributes to tissue damage by promoting mitochondrial dysfunction. Blocking Ang II signalling protects against neurodegenerative processes and promotes longevity in rodents. Altogether these findings open the unanticipated perspective for exploring Ang II signalling in therapeutic interventions in inflammatory diseases and aging-related tissue injury. This review extends from the discovery of Ang II and its implications in renal and cardiovascular physiology to cover the roles of the system in inflammation, tissue injury, autoimmunity, oxidative stress and aging.
PMID: 20597104
It is evident in the decline in immune function that comes with aging.
That most of the researchers at the Conference stand on the edge of better understanding their results in the context of the Th1 pathogens was best exemplified by a presentation given by Rita Effros of UCLA. Effros actually presented data showing that chronic viral infection leads to an increase in senescent cells as well as significant decreases in telomere length. Effros stressed how the constant effort that the body must extend in order to try to keep latent viral infections under control clearly detracts from the energy the body needs in order to effectively manage the waste products that damage the tissues. Yet Effros is convinced that chronic viral infections cannot be eliminated and that, once present in a host, they cannot be stopped from causing an inflammatory response for the remainder of a patient’s life.
As Judith Campisi of the Lawrence Berkeley National Library and Buck Institute made clear in a speech, cancerous stimuli often foster the development of senescent cells and, as discussed above, the Th1 pathogens are quite prolific during cancer. But the greatest giveaway that senescent cells are at the mercy of the Th1 pathogens stems from the fact that, as Campisi described, senescent cells are metabolically active and secrete myriad inflammatory cytokines. In my opinion, nothing screams infection more than the release of inflammatory cytokines, since the inflammatory molecules are released by the immune system in response to infection. Campisi also described how, although senescent cells are targeted for clearance by the innate immune system, they are able to defy the immune response by secreting high levels of enzymes called matrix metalloproteinases (MMPs). In my opinion, the creation of these enzymes probably marks yet another way the Th1 pathogens have evolved to alter cellular machinery in order to foster their survival.
Hypothesis: The Aging Paradox and Autoimmune Disease24) Authors: Eyal Talora; Noel R. Rosea
Abstract The demographic imperatives of the 1990s and the coming decades point to a growing need for research on aging. Investigations of the mechanisms underlying age-related decrease in host defenses have been the focus of many clinical studies on immune function of the elderly1-3. Moreover, it is now estimated that 5% of the elderly population suffer from some form of autoimmune disease contributing to increased morbidity and, at times. to increased mortality in the aged population4-10 and that 10-15% of seemingly healthy individuals over the age of 60 have a significant level of autoantibodies in their serum11,12. The incidence of autoimmunity tends to increase in the aged population as compared with race- and sex-matched young adults4,11,12. Some immunological explanations of this phenomenon may include (i) a repeated or prolonged insult by endogenous auto-antigen, or (ii) molecular mimicry with environmental antigens, or (iii) decrease in the generation of suppressor cells.
Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. by Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, Rizzo C, Colonna-Romano G, Lio D, Di Carlo D, Palmas MG, Scurti M, Pini E, Franceschi C, Vasto S Related Articles Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches.
Curr Pharm Des. 2010;16(6):609-18
Authors: Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, Rizzo C, Colonna-Romano G, Lio D, Di Carlo D, Palmas MG, Scurti M, Pini E, Franceschi C, Vasto S
A typical feature of ageing is a chronic, low-grade inflammation characterized by a general increase in the production of pro-inflammatory cytokines and inflammatory markers (“inflamm-ageing”). This status may slowly damage one or several organs, especially when unfavorable genetic polymorphisms and epigenetic alterations are concomitant, leading to an increased risk of frailty together with the onset of age-related chronic diseases. The contribution of different tissues (adipose tissue, muscle), organs (brain, liver), immune system and ecosystems (gut microbiota) to age-related inflammation (“inflamm-ageing”) will be discussed in this review in the context of its onset/progression leading to site-restricted and systemic effects. Moreover, some of the possible strategies and therapies to counteract the different sources of molecular mediators which lead to the age-related inflammatory phenotype will be presented.
PMID: 20388071
Purging Cells in Mice Is Found to Combat Aging IllsBy NICHOLAS WADE In a potentially fundamental advance, researchers have opened up a novel approach to combating the effects of aging with the discovery that a special category of cells, known as senescent cells, are bad actors that promote the aging of the tissues. Cleansing the body of the cells, they hope, could postpone many of the diseases of aging. The findings raise the prospect that any therapy that rids the body of senescent cells would protect it from the ravages of aging. But many more tests will be needed before scientists know if drugs can be developed to help people live longer. Senescent cells accumulate in aging tissues, like arthritic knees, cataracts and the plaque that may line elderly arteries. The cells secrete agents that stimulate the immune system and cause low-level inflammation. Until now, there has been no way to tell if the presence of the cells is good, bad or indifferent.
References
[PMID: 20978262] [DOI: 10.1001/jama.2010.1546]
[PMID: 15141078] [PMCID: 419577] [DOI: 10.1073/pnas.0402268101]
[PMID: 20439540] [PMCID: 2867291] [DOI: 10.1084/jem.20091419]