
This is an old revision of the document!
Table of Contents
Alzheimer's disease and dementia
Meaning “deprived of mind,” dementia is a serious loss of cognitive ability in a previously unimpaired person, beyond what might be expected from normal aging. Alzheimer's disease – also called Alzheimer disease, Senile Dementia of the Alzheimer Type (SDAT) or simply Alzheimer's – is the most common form of dementia.
Recent evidence suggests that infectious agents need serious investigation as potentially important factors that contribute to the progression, complexity and severity of Alzheimer's.1) Perhaps the most striking of this evidence is that amyloid-beta, the protein which builds up in the brains of Alzheimer's patients, is an antimicrobial petide.
Diagnosis
Physicians use the diagnosis of mild cognitive impairment (MCI) to predict which patients have the highest risk of Alzheimer's. Although the rate at which patients go on to develop probable Alzheimer’s disease is relatively high – approximately 10-15% per year2) – the criteria for diagnosis of MCI excludes a lot of patients. In order to be given a diagnosis of MCI, patients must be “in good health,” have “no significant cerebrovascular disease,” and be between 55 and 90.3) These criteria rule out a number of patients sick with chronic disease. In fact, most patients with chronic disease report some level of cognitive dysfunctionThe loss of intellectual functions such as reasoning; memory loss; and other neurological abilities that is severe enough to interfere with daily functioning. and would otherwise easily meet the other criteria for MCI. Furthermore, normal cognitive performance is expected to decline as people age; also, people over 90 are ineligible to be diagnosed with MCI, because any memory loss over that age is expected.
In the clinical setting, Alzheimer's disease is usually diagnosed from the patient history, collateral history from relatives, and clinical observations, based on the presence of characteristic neurological and neuropsychological features and the absence of alternative conditions. Advanced medical imaging with computed tomography (CT) or magnetic resonance imaging (MRI), and with single photon emission computed tomography (SPECT) or positron emission tomography (PET) can be used to help exclude other cerebral pathology or subtypes of dementia. Definitive diagnosis of AD requires histopathologic examination, which is rarely done in life.
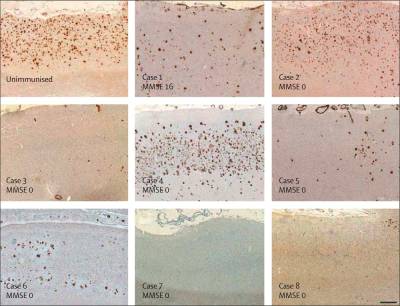
Evidence of infectious cause
- Amyloid-beta, the protein which builds up in the brains of Alzheimer's patients, is an antimicrobial peptide. – See following section
We are excited that someone [the team of Harvard researchers who first identified amyloid beta as an antimicrobial petide] is thinking out of the box in ways that others haven't considered. Most of the evidence about infections and AD has been ignored. The Harvard researchers are going about this in a rational way. It's not such a radical idea. HIV can trigger dementia. Why not other organisms?
Brian Balin, PhD, NeurologyToday
- Bacteria have already been shown to cause dementia and a corresponding deposit of amyloid proteins in a kind of syphilis – It has been known for a century that dementia, brain atrophy and amyloidosis (deposit of amyloid proteins in organs or tissues) can be caused by chronic bacterial infections, namely by Treponema pallidum in the atrophic form of general paresis in syphilis.4)
- Caregivers are more likely to get sick – A subject whose spouse experienced incident dementia onset had a six times greater risk for incident dementia as subjects whose spouses were dementia free.5) The article on familial aggregationOccurrence of a given trait shared by members of a family (or community) that cannot be readily accounted for by chance. discussed the transmissionAn incident in which an infectious disease is transmitted. of microbes and diseases at greater length.
- “Peripheral” infections can hasten the onset and progression of Alzheimer's6) – Cognitive function can be impaired for at least two months after the (apparent) resolution of a systemic infection.7) A 1999 study looked at 22 sets of twins, at least one of whom had been diagnosed with Alzheimer's. Five study participants had serious systemic infection developed Alzheimer's, and they tended to have earlier onset than their corresponding twin.8)
- Peripherally Applied Aβ-Containing Inoculates Induce Cerebral β-Amyloidosis - Intraperitoneal inoculation with β-amyloid–rich extracts induced β-amyloidosis in the brains of β-amyloid precursor protein transgenic mice after prolonged incubation times, possibly indicating that bacteria were co-transported in the extracts.9)
- Classic markers of inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue. are upregulated in Alzheimer's10) – Local brain tissue inflammation in Alzheimer's has been reported to involve upregulated production of classical inflammatory mediators of innate immunityThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease. such as tumour necrosis factor-alpha (TNF-alphaA cytokine critical for effective immune surveillance and is required for proper proliferation and function of immune cells.), interleukin 1 alpha/beta (IL-1 alpha/beta), interleukin 6 (IL-6) and complement proteins that are synthesised locally by cells in the brains of Alzheimer’s patients.11) 12) 13) Some anti-inflammatory therapies have been shown to temporarily delay the progression of Alzheimer's.14)
Specific pathogens already implicated in Alzheimer's
While many more pathogens will likely be identified in patients with Alzheimer's, certain easily cultured and readily identifiable microbes have been repeatedly identified in people with such conditions. According to Urosevic and Martins,15) these include the following viruses: herpes simplex virus 1 (HSV1), human immunodeficiency virus (HIV),16) hepatitis C virus (HCV),17) 18) human herpesvirus 6, cytomegalovirus and others.19) 20)
Strong evidence is available for the presence of intracellular bacterium Chlamydia pneumoniae in brains of AD patients.21) 22) In humans, C. pneumnoiae has been shown to reach the central nervous system via infected mononuclear cells following the breach of blood-brain barrier23) 24) and to induce Alzheimer's-like amyloid deposits in mouse brain upon injection.25) 26)
Finally, infection with Helicobacter pylori has been shown to lead to mild cognitive impairment and may therefore play a role in more severe forms of dementia.27)
Amyloid proteins, the hallmark of Alzheimer's, are produced in response to infection
According to the “amyloid hypothesis,” the lifelong buildup of amyloid-beta (amyloid-β) proteins in the brain is the precipitating factor of Alzheimer's disease.28) 29) Researchers showed that amyloid builds up in the brain before symptoms arise suggesting this hypothesis was true. Further confirmation for this explanation seemed to be offered by the fact that people with Down Syndrome – who have an extra copy of the amyloid beta precursor protein on chromosome 21 – almost universally develop Alzheimer's before the age of 40.30)
At this point, it seemed logical that stopping the production of amyloid-beta would also halt the cognitive decline associated with amyloid buildup. However, in a 2008 Lancet paper, Holmes et al. reported a trial in which an experimental vaccine succeeded in reducing production of amyloids (and the deposition of amyloids in the brain) but had no effect on neurological decline.31) This suggested that while amyloid deposits were associated with the disease, they did not cause it.
In a seminal 2010 study, a team of Harvard researchers showed that amyloid beta can act as an antimicrobial peptide, having antimicrobial activity against eight common microorganisms, including Streptococcus, Staphylococcus aureus, and Listeria.32) This led study author Rudolph E. Tanzi, PhD to conclude that amyloid beta is “the brain's protector.”
The similarities between Abeta [amyloid beta] and antimicrobials had been staring us in the face for decades. Abeta looks in size, structure, and biochemical properties like an antimicrobial peptide [called LL-37]. In fact, we have shown that it is a bonafide antimicrobial peptide.
Rudolph E. Tanzi, PhD, NeurologyToday
If amyloid beta were an antimicrobial, one would expect that suppressing amyloid beta production suppresses innate immunity. Indeed, one study found that genetically modified mice that lack the proteases needed to generate amyloid beta have a 60 percent neonate mortality unless raised in sterile conditions. Notably, four major markers of adaptive immune function were normal in these mice.33) A second finding comes from a clinical study of the drug tarenflurbil, published in 2009 in the Journal of the American Medical Association that was shown to slightly lower amyloid beta production. Most telling is that a side effect of patients taking tarenflurbil is significantly increased rates of infection.34)
Role of the Vitamin D Receptor
According to the Marshall PathogenesisA description for how chronic inflammatory diseases originate and develop., microbes subvert immune activity by suppressing expression of key nuclear receptorsIntracellular receptor proteins that bind to hydrophobic signal molecules (such as steroid and thyroid hormones) or intracellular metabolites and are thus activated to bind to specific DNA sequences which affect transcription. involved in innate immune activity such as the Vitamin D ReceptorA nuclear receptor located throughout the body that plays a key role in the innate immune response.. The fact that over-production of amyloid suppresses the VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response. suggests a role of VDR dysfunction in the Alzheimer's disease process. Indeed, a 2010 study in Journal of Alzheimer's Disease showed that the amyloids trigger neurodegeneration not only by inducing LVSCC A1C expression and NGF levels, but also by dramatically suppressing VDR expression.35)
Roles of Brain Angiotensin II in Cognitive Function and Dementia. 36)
Other immune suppression
The most serious health endpoints that have been reported to be associated with extremely low frequency (ELF) and/or radiofrequency radiation (RFR) include childhood and adult leukemia, childhood and adult brain tumors, and increased risk of the neurodegenerative diseases, Alzheimer’s and amyotrophic lateral sclerosis (ALS).
Is the Marshall Protocol applicable for dementia patients?
Int J Cardiol. 2016 Oct: ACEIs and ARBs may effectively prevent all-cause dementia, particularly VD, in patients with type 2 DM and hypertension. Moreover, compared with ACEIs, ARBs appear to be more advantageous in dementia prevention. 37)
There is a clinic in Germany which is treating dementia with a variation of the MP and has found the patients herx to indicate a Th1 diseaseAny of the chronic inflammatory diseases caused by bacterial pathogens.. However, by the time the disease becomes advanced, they are no longer able to look after themselves, and the MP requires a lot of self-discipline in order to be effective.
The problem is compliance, as the patients have trouble focusing on avoiding sunlight/Vit-D, and remembering to take their Benicar. They seem to be responding. And, most important, they herx, their disease relapses after a day soaking in the sun, and some show (very) tentative signs of recovery.
There is not much to be gained by varying from the base MP. With the dementia patients it is tough to get 100% compliance, unless they have an efficient caregiver (in other words, they don't take their meds on time). Additionally, there is little appreciation, at the level of the patient, of avoiding ingested Vitamin D.
There is little chance that a patient having gone through the MP is likely to develop dementia, but at this point it is also unlikely that Alzheimer's patients, in an advanced state of disease, would be able to discipline themselves enough for recovery.
Trevor Marshall, PhD
Published in AJH February 2017 ARB protects Alheimer's
Shuko Takeda & Ryuichi Morishita Angiotensin Receptor Blocker protects Alzheimer's Disease Brain from Ischemic Insult
Role of lifestyle factors
A 2011 study used a mathematical model to surmise that modifiable conditions such as physical inactivity, smoking, depression, low education, hypertension, obesity and diabetes are responsible for about half of the roughly 5.3 million Alzheimer’s cases in the United States and 34 million cases worldwide.38) They went on to conclude that reducing the prevalence of these risk factors by 10 percent could prevent 1.1 million cases worldwide; reducing these risk factors by 25 percent, they claimed, could prevent more than three million cases.
This study fails to consider that such “lifestyle choices” may be due to other factors entirely. A patient with artificially controlled hypertension or diabetes or is a patient who smokes and is unable to quit is not the same as a healthy patient.
Other antimicrobial therapies
Epidemiology and costs to society
Alzheimer’s disease affects approximately 4.5 million people in the U.S. and this number will increase as the population ages and the life-span increases. It is projected in 50 years, as the population ages and the life-span increases, AD will afflict approximately 14 million people.41) Two-thirds of nursing home residents have dementias.42)
Dementia, and specifically Alzheimer's disease, may be among the most costly diseases for society in Europe and the United States,43) the greatest of which is long-term healthcare. Numbers vary between studies but dementia costs worldwide have been calculated around $160 billion,44) while costs of Alzheimer in the United States may be $100 billion each year.45)
Read More
Notes and comments
<DiseaseHierarchy>
- Legacy content
From: wrotekDate: 2011-08-07 00:57:22 Reply: http://www.marshallprotocol.com/reply.php?topic_id=13678
New paper about spirochetal microbiome in Alzheimer disease
Abstract
http://www.jneuroinflammation.com/content/8/1/90/abstract
full paper
http://www.jneuroinflammation.com/content/pdf/1742-2094-8-90.pdf
Bar graphs,in the end of this paper show
that they have found other spirochaetes to be more prevalent, borrelia wasn't dominant.
Video of oral spirochetes
http://www.youtube.com/watch?v=oHETTEXpwu4
Lol is dental plaque a bacterial biofilm A structured community of microorganisms encapsulated within a self-developed protective matrix and living together. ?
From: edj2001Date: 2011-08-07 15:00:34 Reply: http://www.marshallprotocol.com/reply.php?topic_id=13678
Chronic Inflammation and Amyloidogenesis in Alzheimer’s Disease: Putative Role of Bacterial Peptidoglycan, a Potent Inflammatory and Amyloidogenic Factor
http://www.lymenet.de/shgs/dietmars/ChronicInflammation.pdf
Summary Glycosaminoglycans (GAGs), which occur in most organisms from bacteria to vertebrates, appear to be present in all amyloid deposits, and may play an essential role in the pathogenesis of amyloidosis. It is well established that senile plaques are the sites of chronic inflammation, but the factor activating the complement remain unknown. Here, we analyzed whether the amyloidogenic bacterial peptidoglycan, a potent activator of complement and of the GAG metabolic system is present in senile plaques. Neuropathologically analyzed 54 autopsied brains were investigated. The 54 cases consisted of 32 Alzheimer's disease (AD) cases with severe AD-type changes, 12 cases with low number of senile plaques and 10 cases without any AD-type changes. We have found that in the 32 AD cases with high number of plaques and in the 12 cases with low number of plaques, bacterial peptidoglycan was immunolocalized to senile plaques and on serial sections co-localized with betaamyloid protein. Bacterial peptidoglycan has a variety of biological actions in mammals. It is an inflammatory cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. inducer, activates complement of the classic pathway, affects vascular permeability, generates nitric oxide, induces proteoglycan synthesis and apoptosis, in addition is amyloidogenic. It is well established that all these processes are implicated in AD, suggesting that bacteria or bacterial debris may be one among probably several factors which may trigger the cascade of events leading to chronic inflammation and amyloid deposition in AD.
Key words: Alzheimer’s disease, bacteria, bacterial peptidoglycan, beta-amyloid, chronic inflammation,
Joint Bone Spine. 2010 Jul;77(4):366-7. Epub 2010 May 15. Improvement of cognition, a potential benefit of anti-TNF therapy in elderly patients with rheumatoid arthritis. Chen YM, Chen HH, Lan JL, Chen DY. PMID: 20478733
Mosconi, L., J. O. Rinne, et al. (2010). “Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer's.” Proc Natl Acad Sci U S A. 20231448
Having a parent affected with late-onset Alzheimer's disease (LOAD) is a major risk factor among cognitively normal (NL) individuals. This (11)C-Pittsburgh Compound B (PiB)-PET study examines whether NL individuals with LOAD parents show increased fibrillar amyloid-beta (Abeta) deposition, a hallmark of Alzheimer's disease (AD) pathology and whether there are parent-of-origin effects. Forty-two 50- to 80-year-old NL persons were examined with PiB-PET. These individuals included 14 NL subjects with a maternal family history (FH) of LOAD (FHm), 14 NL subjects with a paternal FH (FHp), and 14 NL subjects with a negative family history of any dementia (FH-). Statistical parametric mapping and automated regions-of-interest were used to compare cerebral-to-cerebellar PiB standardized uptake value ratios, reflecting fibrillar Abeta burden, across groups. FH groups did not differ in age, gender, education, and apolipoprotein E (ApoE) status. NL FHm subjects showed higher PiB retention in AD-affected anterior and posterior cingulate cortex (PCC), precuneus, parietal, temporal, occipital, and frontal cortices, right basal ganglia, and thalamus, compared with FH- and FHp subjects. FHp subjects showed increased PiB retention in the PCC and frontal cortex, intermediate between FHm and FH- subjects. Results remained significant after controlling for age, gender, education, and ApoE status. Children of parents with LOAD, particularly those with affected mothers, have increased fibrillar Abeta load in AD-vulnerable regions compared with controls, perhaps accounting for the known increased risk for AD. Present findings may motivate further research on familial transmission and parent-of-origin effects in LOAD.
Lots of potential stuff here:
http://www.alzforum.org/res/for/journal/balin/default2.asp
http://www.alzforum.org/res/for/journal/balin/default.asp
Live Discussion: The Pathogen Hypothesis
Updated 12 March 2009
We held this live discussion with discussants Brian Balin, Denah Appelt, Joseph Lyons, Ruth Itzhaki, and Curtis Dobson on 1 July 2004.
See Transcript—Posted 6 August 2004 See comment by Stephen R. Robinson—Posted 1 July 2004 See Q&A—Updated 30 July 2004
Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging 25(4);619-627. Abstract Little CS, Hammond CJ, MacIntyre A, Balin BJ, Appelt DM. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging 2004;25(4);419-429. Abstract Robinson SR, Dobson C, Lyons J. Challenges and directions for the pathogen hypothesis of Alzheimer's disease. Neurobiol Aging 2004; 629-637. Abstract
Moderator’s summary: Pathogens as a cause of Alzheimer’s disease
By June Kinoshita
The notion that microbes such as herpes simplex virus 1 (HSV1) and Chlamydophila pneumoniae (Cp) could be a causal factor in Alzheimer’s diseases would probably be viewed by the main stream of AD researchers as being beyond the pale. Although a small body of recent findings has reported strikingly strong associations between these pathogens and AD [1,7], subsequent attempts to replicate the findings have met with mixed results (discussed in [10]). At this juncture, it might be convenient to dismiss the hypothesis, but as both sides of this debate session agreed, there are plausible reasons for these discrepancies that deserve to be resolved through further research. While opinions diverged on the strength of evidence for and against the hypothesis, there was a consensus that the possibility of common infectious agents causing such a widespread scourge of old age is one that is too important to ignore.
It may be of interest to step back from the specific merits and future challenges of the pathogen hypothesis (which the participants cover thoroughly in this issue) to ponder changes in the medical culture that may have contributed to the willingness of the debate participants and audience alike to weigh the evidence dispassionately rather than to dismiss the whole idea as being implausible on the face of it. Most researchers today grew up in an era when microbes were presumed to have been brought under human control. The 19th and 20th centuries saw the microbial perpetrators of the great killer diseases tracked down one by one and vanquished with drugs and vaccines. Events over the past two decades have rudely awakened medical science to the reality that we have not, after all, advanced into the post-infectious era. The AIDS pandemic and emergence of drug-resistant tuberculosis, malaria and other scourges shocked us into realizing that microorganisms have been far from conquered. These devastating setbacks have driven home the fact that we are engaged in an evolutionary arms race in which our science and wits are pitted against the ability of microbes to adapt to our most clever weapons.
In the same period, a microbe, Helicobacter pylori, came to be accepted as causing duodenal ulcers and gastric cancers [3]. Previously, ulcers were viewed as a classic degenerative condition, the result of some toxic combination of stress, chemical irritants and bad genes. The discovery of a bacterial origin was greeted initially with hostility, but was eventually hailed as marking a paradigm shift in the pathogenesis of chronic diseases. More recently, another microbe, C. pneumoniae, has come under suspicion for playing a role not only in AD, but in atherosclerosis [2,5], the preeminent chronic killer disease.
These may not be flukes, argues biologist Paul Ewald. Ewald has championed a theory, first suggested by physicist Gregory Cochran, that most, if not all, of the chronic degenerative diseases of aging are microbial in origin [4]. While a great deal of effort is currently being invested in pinpointing genes for late-onset Alzheimer disease, the evolutionary argument holds that deleterious genetic mutations, even those that are expressed late in life, cannot persist in a population. Pathogens, in contrast, can persist indefinitely because the host’s ability to evolve resistance to pathogens is matched by the pathogens’ ability to keep shifting their strategy for living off the host. Thus, Ewald writes, “If we see chronic diseases that have commonly been causing damage for a long time, the best bet is that they have infectious causes” [6].
Evolutionary theory has yet to make inroads into the thinking of most Alzheimer researchers. For the majority, it is safe to assume that the idea that microorganisms can cause Alzheimer’s stretches credulity. If pathogens were responsible, one might well wonder how the culprits could have escaped the scrutiny of generations of pathologists. Microbes, however, are capable of astounding stealth. They can insinuate themselves into host cells and genomes, where they may lie latent and be very challenging to detect. Microbes can also leave a trace in the host’s immune memory, exerting lethal effects not through acute infection but by triggering autoimmuneA condition or disease thought to arise from an overactive immune response of the body against substances and tissues normally present in the body responses through molecular mimicry between microbial proteins and host proteins [12].
While these are some compelling theoretical arguments for taking the pathogen hypothesis seriously, the burden lies with proponents to prove the theory, rather than with the indifferent majority to disprove it. In this issue of Neurobiology of Aging, Itzhaki et al. [7], present arguments in support of the pathogen hypothesis, reviewing not only the positive and negative studies that have sought evidence for HSV1 and Cp in Alzheimer brains, but also discussing how pathogens might interact with other known risk factors for AD, such as APOE-α 4 genotype [8], aging, the immune system and trafficking of pathogens into the central nervous system. The companion article by Robinson et al. [10], points out some important discrepancies in these studies and discusses major arguments that could be made against the hypothesis, such as whether it is compatible with the existence of inherited forms of AD. The authors also make constructive suggestions regarding future research. Clinical trials of antibiotics or antiviral drugs, for example, could test whether removing a putative pathogen has any effect on disease progression.
As these articles make clear, these are still early days for the pathogen hypothesis, and the proponents have their work cut out for them. Both HSV1 and Cp are highly challenging to detect, and disputes over their association with AD are clouded by methodological issues. A rigorous effort to test the hypothesis would profit from standardizing methods, for example by distributing a uniform set of tissues with positive and negative controls to determine whether all of the laboratories involved are achieving equal levels of sensitivity. The standard protocol should also require multiple testing of each brain [11], preferably using diverse methods.
An animal model that develops AD pathology and behavioral deficits upon exposure to pathogens would help establish the credibility of the hypothesis. The Balin laboratory presented a mouse model that develops amyloid-beta deposits in the brain following intranasal infection with Cp [9]. That study awaits publication and independently replication. Finally, the hypothesis might be more readily accepted if its advocates could clarify whether the two pathogens implicated to date are acting through independent pathways, or are involved in a common mechanism.
At the end of the day, one might ask, so what? Suppose that microbes cause Alzheimer’s, how will that change strategies for treating the disease? Proponents of the hypothesis suggest that antimicrobial drugs or vaccines can be marshaled to nip the disease in the bud. However, if microbes turn out to work harm through amyloid-beta or non-specific inflammatory responses, would not these remain the better therapeutic targets? These questions probably cannot be answered until more is known about whether and how pathogens contribute to AD. Readers of Robinson et al., and Itzhaki et al., in this issue, are invited to consider the facts and issues and decide whether it is worth enlarging their imaginations to include microbes as potential players in causing Alzheimer’s disease.
Note on Permissions: The background text for this live discussion, “Moderator’s summary: Pathogens as a cause of Alzheimer’s disease,” by June Kinoshita, originally appeared in Neurobiology of Aging 25 (2004) 639–640. (See Science Direct). Articles by Robinson et al., Itzhaki et al., and Little et al. reprinted from Neurobiol Aging, 25, with permission from Elsevier.
References [1] Balin BJ, Gerard HC, Arking EJ, Appelt DM, Branigan PJ, Abrams JT, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol (Berl) 1998;187:23– 42. Abstract
[2] Campbell LA, Kuo CC. Chlamydia pneumoniae and atherosclerosis. Semin Respir Infect 2003;18(March (1)):48–54. Abstract
[3] Cilley RE, Brighton VK. The significance of Helicobacter pylori colonization of the stomach. Semin Pediatr Surg 1995;4(November (4)):221–7. Abstract
[4] Cochran GM, Ewald PW, Cochran KD. Infectious causation of disease: an evolutionary perspective. Perspect Biol Med 2000;43 (Spring (3)):406–48. Abstract
[5] de Boer OJ, van der Wal AC, Becker AE. Atherosclerosis, inflammation, and infection. J Pathol 2000;190(3):237–43. Abstract
[6] Ewald P. Plague time. Anchor Books; 2002. p. 56.
[7] Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging 2004; this issue. See .pdf above.
[8] Itzhaki RF, Dobson CB, Lin WR, Wozniak MA. Association of HSV1 and apolipoprotein E-varepsilon4 in Alzheimer’s disease. J Neurovirol 2001;7(December (6)):570–1. Abstract
[9] Little CS, Hammond CJ, MacIntyre A, Balin BJ, Appelt DM. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging 2004;25(4), in press. See .pdf above.
[10] Robinson SR, Dobson C, Lyons J. Challenges and directions for the pathogen hypothesis of Alzheimer’s disease. Neurobiol Aging 2004; this issue. See .pdf above.
[11] Smieja M, Mahony JB, Goldsmith CH, Chong S, Petrich A, Chernesky M. Replicate PCR testing and probit analysis for detection and quantitation of Chlamydia pneumoniae in clinical specimens. J Clin Microbiol 2001;39(May (5)):1796–801. Abstract
[12] Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science 1998;279(February (5355)):1305. Abstract
Additional References
Higuchi Md et al. Trypanosoma cruzi trans-sialidase as a new therapeutic tool in the treatment of chronic inflammatory diseases: possible action against mycoplasma and chlamydia. Med Hypotheses. 2004 Jan 1;63(4):616-23. Abstract
Hill JM, Steiner I, Matthews KE, Trahan SG, Foster TP, Ball MJ. Statins lower the risk of developing Alzheimer's disease by limiting lipid raft endocytosis and decreasing the neuronal spread of Herpes simplex virus type 1. Med Hypotheses. 2005;64(1):53-58. Abstract
Mori I, Kimura Y, Naiki H, Matsubara R, Takeuchi T, Yokochi T, Nishiyama Y. Reactivation of HSV-1 in the brain of patients with familial Alzheimer's disease. J Med Virol. 2004 Aug ; 73(4):605-11. Abstract
Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004 Sep 1;18(5):407-13. Abstract
Comment by Stephen R. Robinson, Monash University, Australia—Posted 1 July 2004
When will the pathogen hypothesis catch on?
The idea that Alzheimer's disease is caused by a pathogen which invades the brain has been around for decades, but this notion has never attracted serious attention from mainstream researchers. It is often dismissed because “if AD was really caused by a virus or bacteria, they would have found it by now, and in any case everyone knows that AD is not contagious—it only affects the aged”. This reasoning overlooks the fact that the vast majority of diseases known to humanity are caused by pathogens, including quite a few that affect cognitive function, either directly (eg. HIV-1) or indirectly (eg. hepatitis). That the pathogen has not yet been identified is hardly surprising. After all, it took thousands of researchers, two decades and many billions of dollars to reach the conclusion that amyloid deposition does not cause AD. The marginalization of the pathogen hypothesis has stymied research in this area, and much of the supporting data which exists was generated on a pauper's budget, doing credit to the tenacity of proponents such as Ruth Itzhaki, Brain Balin and Mel Ball.
Since the leading proponents of the amyloid deposition hypothesis capitulated (Hardy and Selkoe, 2002), the Alzheimer's field has been left in a vacuum. Sure we still have the 'oligomeric amyloid hypothesis' the 'inflammation hypothesis' and the 'oxidative stress hypothesis' but when one looks beyond the hype it is clear that they merely describe a facet of AD, not its cause. They cannot explain for example, the spatiotemporal spread of plaques and tangles, why certain neurotransmitter types are preferentially affected, or why particular pathways in the brain are selectively targeted. They cannot account for the non-cognitive behavioural disturbances (eg sundowning), or the predilection for old age, and they struggle to explain why ApoE4 is the major genetic risk factor.
The pathogen hypothesis by contrast, offers explanations for all facets of AD, and for this reason it deserves serious consideration. The pathogen hypothesis was showcased in a debate at the second Challenging Views of Alzheimer's Disease conference in July, 2003. As an 'outsider' I was astounded to discover that despite three decades of publications by its proponents, not a single skeptic had taken the pathogen hypothesis seriously enough to write a critique. With colleagues Curtis Dobson and Joseph Lyons, we have now written that critique (Robinson et al., 2004). It is clear to us that much research remains to be done before a strong case can be established, yet it is equally evident that many important questions and issues are ripe for investigation. Certainly there are enough indirect observations to pique the interest of any objective researcher, including reports in the past few months of HSV-1 in the brain tissue of AD patients (Denaro et al., 2003; Mori et al., 2004).
Who knows, perhaps one day we will be able to immunize against AD!
References
Denaro, F.J., Staub, P., Colmer, J. and Freed, D.M. (2003) Coexistence of Alzheimer disease neuropathology with herpes simplex encephalitis. Cell Mol Biol (Noisy-le-grand). 49: 1233-40.
Hardy, J., and Selkoe, D.J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 297: 353-6.
Mori, I., Kimura, Y., Naiki, H., Matsubara, R., Takeuchi, T., Yokochi, T. and Nishiyama, Y. (2004) Reactivation of HSV-1 in the brain of patients with familial Alzheimer's disease. J. Med. Virol., 73: 605-11.
Robinson, S.R., Dobson, C. and Lyons, J. (2004) Challenges and directions for the pathogen hypothesis of Alzheimer's disease. Neurobiol. Aging, 25: 629-637.
Q. From Joy K.—Posted 20 July 2004 Has anyone tested the use of antibiotics for Alzheimer's patients? My mother was diagnosed with the disease more than seven years ago. Although she quit after the diagnosis, she was a heavy smoker most of her life, which resulted in congestion problems. Over the last seven years she was given antibiotics several times. Each time her condition improved dramatically. When she stopped the medication she reverted back to the way she was before. She is now in the last stages of her disease and refuses to eat or drink. She was sent to the emergency room and not expected to survive the night. They gave her and antibiotic drip and by the next day she was fighting to go home. She recognized us, was able to put three words together, and understood and responded to everything we said to her. She even played a little joke on my sister, pretending to be dead and then jump up laughing because she scared her.
She has not been this responsive in close to a year! I attribute it to the antibiotic drip. In the past when she took antibiotics orally she significantly improved but the drip seemed to really make a huge difference. I hope something can be done to research this. I am trying to tell everyone I can. Please let me know if this has been researched. Reply from Brian Balin, Ph.D., Philadelphia College of Osteopathic Medicine—Posted 20 July 2004 Remarkably, this is something that has been recognized by clinicians for many, many years. I have innumerable accounts from individuals who have reported on exactly the same response. There have been reports back to me of individuals who have not spoken for years that have “recovered” this ability following antibiotic therapy. Is the response specific to treating an infection systemically or in the brain, or does it have to do with an anti-inflammatory action of the antibiotics? We just don't have the answers to these questions at this time. In my estimation, there has to be a mandate in this for performing clinical trials based on the antibiotic approach. Hopefully, we can convince the NIH or big pharma that these trials would be worthwhile.
Q. by Allen Cox—Posted 30 July 2004 Several Alzheimer's patients have had postmortem studies done and the Lyme spirochete has been found in the brain embedded in neurons. The following web site lists an article by Thomas Grier on Lyme spirochetes in Alzheimer patients. (Here's another link—ARF) Q. by Donna Walraven, MSW—Posted 23 January 2009
When my father was alive there was an occasion where he had a serious bladder infection that was finally treated by a urologist outside of the nursing home where he stayed in Port Lavaca, Texas. The urologist gave him powerful antibiotics. After a few days on these antibiotics my father became lucid for over a week. He did not know my name before; now he was calling me by name again, and not just responding to questions, but actually carrying on a conversation with me.
It was a gift because soon after this episode he was overfed again and had to be resuscitated for the third time, but this time something happened to his throat and he was unable to eat again. He died about 10 days later.
I just thought that someone should know because it did seem to help him for a time.
Objection: ApoE proves that genetics is critically important in late-onset AD.
Balin: Paul Ewald presents a very intriguing argument with regard to chronic disease. He states that “chronic diseases, if they are common and damaging, must be powerful eliminators of any genetic instruction that may cause them.” Thus, he goes on to argue that the only factor capable of staying ahead of natural selection is pathogens, which can mutate their way rapidly around any genetic adaptations. In essence, we are in a genetic arms race against microbes that we cannot win. Mathematical models based on this concept imply that nongenetic factors are almost entirely responsible for chronic degenerative diseases.
What, then, about ApoE? Ewald argues that genetic causes of chronic disease will persist only if a genetic instruction provides a compensating benefit. For example, sickle cell anemia is caused by a genetic mutation that, in heterozygotes, protects against malaria, which kills millions worldwide each year. The relatively few individuals who inherit two copies of the sickle cell gene suffer a painful and crippling disease, but the gene has persisted because it contributes significantly to the survival of the more numerous heterozygotes. It has been hypothesized that the ApoE4 allele evolved late in human evolution and may have conferred a benefit in younger individuals, perhaps by modifying lipid metabolism as the amount of animal fats in the diet increased. The ε4 allele might be one that is beneficial during reproductive years but becomes a detriment in old age.
ApoE4 may confer increased susceptibility to infection in Alzheimer disease. Studies by Dobson and Itzhaki, 1999, demonstrated that AD patients with the ε4 allele were more likely to also harbor HSV1. Furthermore, in a recent study by Gerard et al., 2005, the bacterial load of Chlamydia pneumoniae in the Alzheimer brain was significantly higher in the ApoE4-bearing AD brain tissues, as compared to the AD brain tissues not expressing the ε4 allele. Is it possible that HSV1, Chlamydia pneumoniae, and possibly other infectious microbes could be hijacking molecules for cholesterol delivery to enter the brain? This scenario offers a novel interpretation for how a genetic risk factor and infectious agent might interact to cause disease.
Objection: The risk for late-onset AD is clearly heritable, even in individuals with the ApoE4 allele, so there must be other risk-factor genes waiting to be discovered.
Balin: No doubt, with further analysis, other risk-factor genes will be discovered. However, studies of monozygotic twin populations clearly demonstrate that genetic factors account only partially for an increased risk of AD. Genes predispose individuals to AD, but environmental influences must come into play to ultimately cause disease. Just as previously mentioned, there are a multitude of examples that suggest that the combination of a pathogen and genetic susceptibility can result in disease.
Objection: Most of us harbor pathogens in the CNS with no ill effect.
Balin: There is a presumption that the presence of infectious agents in tissues is not harmful if there is no obvious acute or reactive process. Any microbial invasion of the human central nervous system that results in acute, chronic, and/or permanent residence cannot be good. Of all the tissues in the body, one would expect the central nervous system, which controls our entire being, to be most “sterile.” Ironically, the central nervous system is the one site in the body where infectious agents can persist over a long time because in this part of the body, they can evade immune surveillance by the body's defense system.
However, this begs the question of the brain's “internal immune surveillance” that is accomplished principally by microglia and astroglia. Chronic and/or persistent “turn-on” of these cells due to the presence of infection or products of infection would not be healthy for nerve cells. One could envision a tissue response by the brain that would result in chronic degenerative damage. A perfect analogy is tuberculosis in lung tissue in which chronic inflammation due to a persistent stimulus (i.e., mycobacterium or products of mycobacterium in the macrophages) results in granuloma formation, a focus of inflammation around damaged or dead, necrotic lung tissue. Could the well-circumscribed dense-core amyloid plaque be a comparable “granuloma” of the brain?
Objection: Many neurologists and neuropathologists will object that “since most people have viruses in their nervous systems, the viruses must not be contributing to disease.”
Balin: This argument is flawed. Consider Helicobacter pylori infection in the stomachs of human populations. This organism has been proven in the last two decades of the twentieth century to cause gastric and peptic ulcers, gastric cancer, and mucosal-associated lymphoid tissue tumors in the stomach. Approximately 3.5 billion people are infected with this organism, but only 10 percent show obvious disease. Are we to believe that because such a large population is infected with this organism, but only a small percentage actually fall ill, that the presence of the organism in the gut is of little significance? Obviously, this is not the case.
And, there are parallels in the central nervous system! Herpes viruses that infect neurons are known to cause annoying chronic problems such as cold sores and shingles. Other pathogens are known to cause or contribute to more significant problems such as Bell's palsy and Guillain-Barre syndrome. But there may be still others that may trigger even more severe disease pathogenesis. For example, the autoimmune responses that lead to multiple sclerosis might be triggered by infection, and AIDS dementia results from long-term infection with the HIV-1 virus. And, it just so happens, that HIV-dementia appears to be worsened in those patients who express the ApoE4 allele (Corder et al., 1998)!
Objection: If pathogens cause AD, why don't we see AD brains full of viruses or other microbes?
Balin: Microbes have many tricks for hiding out in tissues. Viruses, for example, can insinuate themselves into host cells' genes and become invisible. Chlamydia pneumoniae is an extremely interesting organism and candidate for numerous diseases. This bacterium is endocytosed into vacuoles within a variety of cell types and may be retained in certain cells indefinitely. Our own studies have determined that infection by this organism into neuroblastoma cells in culture confers an antiapoptotic effect on the cells, thus ensuring infection in a chronic to persistent state. Difficulties in recognizing this type of infection are a major challenge. Changes in gene and antigen expression with persistency have led, at times, to the undetectability of the organism in tissues and culture samples. Furthermore, homology of some bacterial proteins with eukaryotic proteins may result in antigenic mimicry that can incite an autoimmune response without a clearly identified or identifiable infectious component. These are just a few reasons why associating infection with chronic disease or proving that infection is causing chronic disease is so difficult.
Objection: By what mechanism can chronic infection result in a chronic degenerative disease?
Balin: Chronic diseases can result from direct impact of infection on the genetics of the system. For example, cervical cancer, a chronic disease, is caused by the human papilloma virus (HPV). Different strains of this virus code for proteins that directly impact two proteins, the retinoblastoma tumor suppressor protein, and the p53 protein, which is important in DNA repair. The viral proteins block the ability of the eukaryotic proteins to apply breaks to the cell cycle, thus leading to cellular proliferation and cancer.
Another possible mechanism is autoimmunity such as mentioned above. A microbe may express genes that are molecular mimics of a human protein (say, Aβ). The body mounts an immune attack on the microbe, which is cleared from the scene. Rheumatic heart disease is a familiar example of how this could occur. Years later, some trigger (a new infection, or overexpression of Aβ?) results in an immune attack against the endogenous protein and causes severe tissue damage.
Objection: People have searched in good faith, but the data are not convincing. Ergo, there's nothing to this hypothesis.
Balin: Several studies, including our own, have reported a remarkably strong association between a microbe and AD, but other studies have not shown such an association. There are numerous reasons why there are discrepancies in the different studies. These include tissue sampling, use of different polymerase chain reaction (PCR) primers and probes, and different antibody probes to different antigenic determinants of the organism at different stages of infection. We must work on standardizing approaches to resolve some of these issues. Numerous laboratories have applied real-time PCR approaches in this effort. However, even in these instances, newer findings on gene expression and gene modifications found in microorganisms are clouding these approaches. Thus, numerous techniques and collaborative approaches are required to objectively address these issues prior to ruling out infectious risk and/or causation of chronic diseases. The solution is still unfolding in the laboratory, where increasingly sophisticated techniques are being used to discover if infection is involved in the pathogenic processes. Evolution of newer techniques and development of animal models of infection are some of the key reasons we can now more efficiently investigate pathogen causation in chronic diseases.
Objection: Why mess around with microbes when there are other, more compelling ideas, such as the amyloid cascade hypothesis, with so much more supporting data, including persuasive genetic findings on the cause of early-onset familial AD and the increased risk of LOAD associated with ApoE4?
Balin: Until we can clearly determine the cause of AD and other neurodegenerative diseases, as well as many other chronic diseases, infection must be included as a hypothesis. In the history of medicine and science, this has proven to be and continues to be the greatest determinant of disease. Researchers must ask themselves the following questions: 1) Has all previous work on a given chronic disease of aging demonstrated that infection is not involved in the disease? 2) Have we put enough resources behind the investigators and investigations studying infection in chronic diseases of aging to rule out infection as causing the problems? And last, but not least, 3) do we currently have enough knowledge of infectious diseases in chronic conditions to proclaim that chronic infection does not cause chronic disease? If the answers to any of these questions is no, then pathogen involvement in chronic diseases of aging has to be given high priority for consideration.
The possibility that treatable common microbial infections are contributing to the global crisis of Alzheimer disease has implications for public health that are too important to ignore. A thorough review of many of the issues discussed here can be found through the American Academy of Microbiology. A report entitled “Microbial Triggers of Chronic Human Illnesses” was compiled from a colloquium held in June 2004. This report highlights many features relevant to this discussion. For example, two sections report on host factors and microbial factors that contribute to illness. Host factors that are considered are: genetics, concomitant infections, age, dose, gender, hormonal factors, immune status, nutritional status, behavioral factors, and exposure to non-infectious agents. Microbial factors considered are: viral or infectious genetic integration into host genome, latency factors, ability to bind to mucosal surfaces or other tissues, characteristics of the target organ, high mutation rate, and immune evasion. In addition, the report highlights currently available techniques for the detection of pathogens with an analysis of the technical strength and weakness.
In summary, this report provides excellent insight into current strategies and the required needs for addressing microbial triggers of chronic illnesses such as Alzheimer disease.
The Effects of Vitamin D Receptor Silencing on the Expression of LVSCC-A1C and LVSCC-A1D and the Release of NGF in Cortical Neurons.
http://www.ncbi.nlm.nih.gov/pubmed/21408608
CONCLUSIONS/SIGNIFICANCE: Our results indicate that suppression of VDR disrupts LVSCC-A1C and NGF production. In addition, when VDR is suppressed, neurons could be vulnerable to aging and neurodegeneration, and when combined with Aβ toxicity, it is possible to explain some of the events that occur during neurodegeneration.
esearchers from Boston University School of Medicine (BUSM) have found that angiotensin receptor blockers (ARBs) – a particular class of anti-hypertensive medicines – are associated with a striking decrease in the occurrence and progression of dementia. These findings appear in the January issue of the British Medical Journal http://www.physorg.com/news136426165.html
A Novel Perspective for Alzheimer's Disease: Vitamin D Receptor Suppression by Amyloid-β and Preventing the Amyloid-β Induced Alterations by Vitamin D in Cortical Neurons
http://iospress.metapress.com/content/mg838747x7844354/
“Our results showed that the A triggers neurodegeneration not only by inducing LVSCC A1C expression and NGF levels and but also by dramatically suppressing VDR expression. ”
Amyloid-β (Aβ) is the core component of amyloid plaques of Alzheimer's disease (AD). The effects of Aβ include damage to neuronal plasma membrane, disruption of Ca2+ homeostasis, and alterations of neurotrophic factor levels. The aim of this study was to determine the effects of Aβ treatment on vitamin D receptor (VDR), L-type voltage sensitive calcium channels A1C (LVSCC A1C), NGF, and observing the effects of vitamin D treatment on Aβ induced alterations in primary cortical neurons. As to the latter, we aimed to test the suggested neuroprotective role of vitamin D as a neglected neurosteroid. The expressions of VDR and LVSCC A1C were studied with qRT-PCR and Western blotting. NGF and cytotoxicity levels were determined by ELISA. Apoptotic cell death was investigated with caspase-3 protein expression by Western blotting. Our results showed that the Aβ triggers neurodegeneration not only by inducing LVSCC A1C expression and NGF levels and but also by dramatically suppressing VDR expression. Administration of vitamin D to this model protected neurons by preventing cytotoxicity and apoptosis, and also by downregulating LVSCC A1C and upregulating VDR. Additionally, vitamin D brought NGF expression to a state of equilibrium and did not show its apoptosis inducing effects. Consequently, prevention of Aβ toxicity which was one of the major component of AD type pathology by vitamin D treatment and understanding how Aβ effects vitamin D related pathways, might open up new frontiers in clarifying molecular mechanisms of neurodegeneration and provide basis for novel perspectives in both preventing and treating AD.
Put in link between Alzheimer's and autism regarding amyloid-beta?
Fluids Barriers CNS. 2011 Jul 8;8(1):20. [Epub ahead of print] 1a,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-b peptide(1-40) from mouse brain across the blood-brain barrier. Ito S, Ohtsuki S, Nezu Y, Koitabashi Y, Murata S, Terasaki T. Abstract ABSTRACT: BACKGROUND: Cerebrovascular dysfunction has been considered to cause impairment of cerebral amyloid-b peptide (Ab) clearance across the blood-brain barrier (BBB). Further, low levels of vitamin D are associated with increased risk of Alzheimer's disease, as well as vascular dysfunction. The purpose of the present study was to investigate the effect of 1a,25-dihydroxyvitamin D3 (1,25(OH)2D3), an active form of vitamin D, on cerebral Ab clearance from mouse brain. METHODS: The elimination of [125I]hAb(1-40) from mouse brain was examined by using the Brain Efflux Index method to determine the remaining amount of [125I]hAb(1-40) radioactivity after injection into the cerebral cortex. [125I]hA(1-40) internalization was analyzed using conditionally immortalized mouse brain capillary endothelial cells (TM-BBB4). RESULTS: Twenty-four hours after intraperitoneal injection of 1,25(OH)2D3 (1 ug/mouse), [125I]hAb(1-40) elimination from mouse brain was increased 1.3-fold, and the level of endogenous Ab(1-40) in mouse brain was reduced. These effects were observed at 24 hr after intraperitoneal injection of 1,25(OH)2D3, while no significant effect was observed at 48 or 72 hrs. Vitamin D receptor (VDR) mRNA was detected in mouse brain capillaries, suggesting that 1,25(OH)2D3 has a VDR-mediated genomic action. Furthermore, forskolin, which activates mitogen-activated protein kinase kinase (MEK), enhanced [125I]hAb(1-40) elimination from mouse brain. Forskolin also enhanced [125I]hAb(1-40) internalization in TM-BBB4 cells, and this enhancement was inhibited by a MEK inhibitor, suggesting involvement of non-genomic action. CONCLUSIONS: Active form of vitamin D, 1,25(OH)2D3, appears to enhance brain-to-blood Ab(1-40) efflux transport at the BBB through both genomic and non-genomic actions. Compounds activating these pathways may be candidate agents for modulating Ab(1-40) elimination at the BBB. PMID: 21740543
Immunohistological detection of Chlamydia pneumoniae in the Alzheimer's disease brain. http://www.ncbi.nlm.nih.gov/pubmed/20863379
Anti-C. pneumoniae antibodies, obtained commercially, identified both typical intracellular and atypical extracellular C. pneumoniae antigens in frontal and temporal cortices of the AD brain. C. pneumoniae, amyloid deposits, and neurofibrillary tangles were present in the same regions of the brain in apposition to one another. Although additional studies are required to conclusively characterize the nature of Chlamydial immunoreactivity in the AD brain, these results further implicate C. pneumoniae infection with the pathogenesis of Alzheimer's disease.
Mol Neurodegener. 2009 Jul 14;4:28. The interactome of the amyloid beta precursor protein family members is shaped by phosphorylation of their intracellular domains. Tamayev R, Zhou D, D'Adamio L. Source Department of Microbiology and Immunology, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461, USA. ldadamio@aecom.yu.edu. Abstract
ABSTRACT:
BACKGROUND:
Brain tissue from patients with Alzheimer's disease has shown an increase of phosphorylation of Tyr-682, located on the conserved Y682ENPTY motif, and Thr-668 residues, both in the intracellular domain (AID) of amyloid beta precursor protein (APP), although the role of these two residues is not yet known.
RESULTS:
Here, we report that the phosphorylation status of Tyr-682, and in some cases Thr-668, shapes the APP interactome. It creates a docking site for SH2-domain containing proteins, such as ShcA, ShcB, ShcC, Grb7, Grb2, as well as adapter proteins, such as Crk and Nck, that regulate important biological processes, cytosolic tyrosine kinases, such as Abl, Lyn and Src, which regulate signal transduction pathways, and enzymes that control phosphatidylinositols levels and signaling, such as PLC-gamma. At the same time, it either reduces (like for JIP1, NUMB, NUMBL and ARH) or abolishes (like for Fe65, Fe65L1 and Fe65L2) binding of other APP interactors. Phosphorylation of Thr-668, unlike Tyr-682, does not seem to affect APP's ability to interact with the various proteins, with Pin1 and X11 being the exclusions. We also found that there are some differences between the interactions to AID and to ALID1 and ALID2, its two homologues.
CONCLUSION:
Our data indicates that APP can regulate diverse cellular processes and that, vice versa, a network of signaling events can impact APP processing. Our results also suggest that phosphorylation of the APP Intracellular Domain will dramatically shape the APP interactome and, consequently, will regulate APP processing, APP transport and APP/AID-mediated functions.
PMID: 19602287 [PubMed - in process]
Am J Pathol. 2011 Nov;179(5):2551-8. Epub 2011 Sep 15.High-resolution 3D reconstruction reveals intra-synaptic amyloid fibrils. Capetillo-Zarate E, Gracia L, Yu F, Banfelder JR, Lin MT, Tampellini D, Gouras GK. Source Department of Neurology and Neuroscience, Weill Cornell Medical College, New York, New York, USA. Abstract β-Amyloid (Aβ) accumulation and aggregation are hallmarks of Alzheimer's disease (AD). High-resolution three-dimensional (HR-3D) volumetric imaging allows for better analysis of fluorescence confocal microscopy and 3D visualization of Aβ pathology in brain. Early intraneuronal Aβ pathology was studied in AD transgenic mouse brains by HR-3D volumetric imaging. To better visualize and analyze the development of Aβ pathology, thioflavin S staining and immunofluorescence using antibodies against Aβ, fibrillar Aβ, and structural and synaptic neuronal proteins were performed in the brain tissue of Tg19959, wild-type, and Tg19959-YFP mice at different ages. Images obtained by confocal microscopy were reconstructed into three-dimensional volumetric datasets. Such volumetric imaging of CA1 hippocampus of AD transgenic mice showed intraneuronal onset of Aβ42 accumulation and fibrillization within cell bodies, neurites, and synapses before plaque formation. Notably, early fibrillar Aβ was evident within individual synaptic compartments, where it was associated with abnormal morphology. In dendrites, increasing intraneuronal thioflavin S correlated with decreases in neurofilament marker SMI32. Fibrillar Aβ aggregates could be seen piercing the cell membrane. These data support that Aβ fibrillization begins within AD vulnerable neurons, leading to disruption of cytoarchitecture and degeneration of spines and neurites. Thus, HR-3D volumetric image analysis allows for better visualization of intraneuronal Aβ pathology and provides new insights into plaque formation in AD. Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved. PMID: 21925470

