Related article: Innate immune response and Th1 inflammation

This is an old revision of the document!
Table of Contents
Metabolism of vitamin D and the Vitamin D Receptor
A number of studies have suggested that patients with chronic inflammatory diseases are deficient in 25-hydroxyvitamin-DThe vitamin D metabolite widely (and erroneously) considered best indicator of vitamin D "deficiency." Inactivates the Vitamin D Nuclear Receptor. Produced by hydroxylation of vitamin D3 in the liver. (25-DThe vitamin D metabolite widely (and erroneously) considered best indicator of vitamin D "deficiency." Inactivates the Vitamin D Nuclear Receptor. Produced by hydroxylation of vitamin D3 in the liver.) and that consuming greater quantities of vitamin D, which elevates 25-D levels, alleviates symptoms of disease. Some years ago, molecular biology identified 25-D as a secosteroid. Secosteroids would typically be expected to depress inflammationThe complex biological response of vascular tissues to harmful stimuli such as pathogens or damaged cells. It is a protective attempt by the organism to remove the injurious stimuli as well as initiate the healing process for the tissue., which is in line with the reports of symptomatic improvement. The simplistic first-order mass-action model used to guide the early vitamin studies has given way to a more complex description of action. When active, the Vitamin D nuclear receptorA nuclear receptor located throughout the body that plays a key role in the innate immune response. (VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response.) affects transcription of at least 913 genes and impacts processes ranging from calcium metabolism to expression of key antimicrobial peptidesBody’s naturally produced broad-spectrum antibacterials which target pathogens..
Located in the nucleus of a variety of cells including immune cells, the VDR is a control system of sorts. When exposed to infection and damage, especially that which is caused by pathogens, the body begins to convert the inactive form 25-D into the active form, 1,25-DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol.. As cellular concentrations of 1,25-D increase, 1,25-D activates the VDR, turning on any number of genes the receptor transcribes.
Hormonal changes result from change in 1,25 dihydroxyvitamin-D
According to a 2010 analysis, the VDR significantly affects 229 human genes. Many of these genes have long been associated with autoimmune diseases and cancers including, for example, the genes IRF8 (linked to multiple sclerosis), and PTPN2 (connected to Crohn's disease and type I diabetes).1) The activation of certain genes also leads to the synthesis of antimicrobial peptides. The antimicrobial peptides are the body's “natural antibiotics” and have a potent anti-bacterial effect.
However, bacteria create ligands, which like 25-D, inactivate the VDR and, in turn, the innate immune responseThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease.. This allows the microbes to proliferate. In response, the body increases production of 1,25-D from 25-D, leading to one of the hallmarks of chronic inflammatory disease: a low 25-D and a high 1,25-D.
This pattern is a result of the disease process rather than a cause. For a variety of reasons, neither increased consumption of vitamin D nor the body's synthesis of additional 1,25-D is ultimately effective at combatting infection.
Vitamin D Receptor (VDR) controls innate immunity
Related articles: Metabolism of vitamin D and the Vitamin D Receptor , Aiming at health
Regulating the VDR
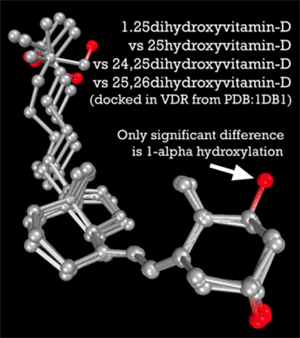
A general appreciation for how 25-D and 1,25-D compete for nuclear receptorsIntracellular receptor proteins that bind to hydrophobic signal molecules (such as steroid and thyroid hormones) or intracellular metabolites and are thus activated to bind to specific DNA sequences which affect transcription. gets to the heart of their opposing roles in the body. According to the Marshall PathogenesisA description for how chronic inflammatory diseases originate and develop., the VDR is foremost a control system. Under most circumstances, the active form, 1,25-D, acts as the “on” switch and the inactive form, 25-D, is the “off” switch.2) 25-D is not completely inactive, but it does not and cannot activate the VDR. As Leow states in Respirology, “25-D levels are not associated with levels of cathelicidin Family of antimicrobial peptides found primarily in immune cells and transcribed by the Vitamin D Receptor. or beta-defensin An antimicrobial peptide found primarily in immune cells and transcribed by the Vitamin D Receptor.-2 [antimicrobial proteins transcribed by the VDR].”3)
Further underscoring this role for these two D metabolites is that 25-D and 1,25-D “happen” to share similar binding affinities for the VDR. According to molecular modeling by Trevor Marshall, PhD, 1,25-D has an affinity of 8.48 (as measured by nanomolar Kd) and 25-D has an affinity of 8.36.4) It would seem that activation of the Vitamin D nuclear receptor is achieved by a delicate balance between the concentrations of a number of endogenous hormones. Indeed, at the risk of overgeneralization, the body increases and decreases the production of 1,25-D to control the innate immune response.
As mentioned before, exposure to injury and infection enhances production of 1,25-D, which in turn leads to the creation of antimicrobial peptides and activation of TLR2A receptor which is expressed on the surface of certain cells and recognizes native or foreign substances and passes on appropriate signals to the cell and/or the nervous system..
However, certain feedback mechanisms are also in place, which allow the body to limit the production of 1,25-D to just that amount needed for proper transcriptional activation of the VDR.
- When the VDR is activated, it transcribes the gene for the enzyme CYP24A1, which increases conversion of 1,25-D into inactive metabolites.
- An activated VDR also controls 1,25-D concentration by limiting transcription of the gene CYP27B1, which converts 25-D into 1,25-D.5)
Bacteria and the VDR
The Antimicrobial Peptide Database lists hundreds of antimicrobial peptides known to kill or inhibit the reproduction of bacteria,6) 793 AmPs found in animals as of January 5, 2009. The sheer diversity of these proteins coupled with the fact that they have been conserved over millenia suggests that enough pathogenic bacteria exist in sufficient quantities to warrant the evolution of these defense mechanisms. It would seem that is in the strong interest of the human body to destroy or disrupt these bacteria.
Pathogenic bacteria are likewise driven by evolutionary impetus: it's in their interest to disrupt the proteins, which interfere with their growth. In what way or ways could bacteria interrupt production of the AmPs?
According to one researcher, it is nearly impossible for bacteria to develop resistance to the AmPs:
Acquisition of resistance by a sensitive microbial strain against antimicrobial peptides is surprisingly improbable.
Michael Zasloff 7)
However, what if it were possible to disrupt the expression of the Vitamin D Receptor by secreting ligands, which bind to and inactivate the receptor? Such bacteria would have an undeniable reproductive advantage.
Think about this for a minute – if you were a persistent pathogen, wouldn’t it seem a good idea to disable your host’s ability to produce antimicrobial peptides? And if you discovered that disabling just one receptor, the VDR, would get rid of both cathelicidin and defB2, wouldn’t you try to evolve a mechanism for doing that?
Trevor Marshall, PhD
Bacteria disable the VDR
In the arms race of host–microbe co-evolution, successful microbial pathogens have evolved ingenious ways to evade host immune responses.8)
Studies have indicated that the dysregulation of VDR may lead to exaggerated inflammatory responses, raising the possibility that defects in Vitamin D and VDR signaling transduction may be linked to bacterial infection and chronic inflammation. Further characterization of Vitamin D/VDR will help elucidate the pathogenesis of various human diseases and in the design of new approaches for prevention and treatment.
Jun Sun 9)
Since the VDR is at the heart of the innate immune system, bacteria can survive by discovering how to disable it through a variety of different actions. Actions accumulate and are more powerful than individual actions.
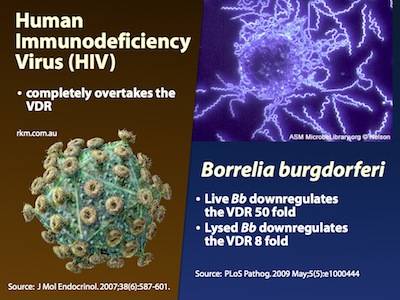
In keeping with evolutionary theory, a growing number of substances and species have been shown to downregulate the activity of the VDR:
- Borrelia burgdorferi – Live Borrelia burgdorferi reduced VDR expression in monocytes (phagocytes) by 50 times, and lysates (“dead” Borrelia) reduced it by 8 times10)
- Mycobacterium tuberculosis enzyme involved in vitamin D and 7-dehydrocholesterolA cholesterol precursor manufactured by humans. When exposed to ultraviolet light converted into vitamin D3. Also known as previtamin-D3. metabolism 14)
- Mycobacterium leprase – produces microRNA-21 to target multiple genes associated with the VDR15)
- “Gliding” biofilm A structured community of microorganisms encapsulated within a self-developed protective matrix and living together. bacteria have been shown to create Capnine – Capnine is a 2-amino-3-hydroxy-15-methylhexadecane-1-sulfonic acid, and is created by the genera Cytophaga, Capnocytophaga, Sporocytophaga, and Flexibacter.16) 17) The secretion of capnine meets an important evolutionary need for bacteria. Capnine possesses a high affinity for the VDR as evidenced by the fact that molecular modeling shows that its stable in the ligand binding pocket. Molecular modeling further shows that when capnine is docked in the VDR, it inactivates the receptor.
- Chlamydia trachomatis – shown to downregulate the VDR in unpublished work
The following substances reduce the number of VDR, without which immune function is limited:
According to the Marshall Pathogenesis, pathogens' production of ligands, which bind to and antagonize (inactivate) the Vitamin D Receptor, is one of the fundamental processes by which chronic inflammatory disease occurs. The consumption of other immunosuppressive substances also has an effect.
One promising area for future research is to fully characterize the breadth and diversity of proteins created by bacteria in infected cells.
Murine models of VDR offer some confirmation
One can see the effects of a dysfunctional VDR in knockout mice, mice genetically engineered to be born without the receptor. These mice demonstrate what it is like to have a VDR completely blocked by bacterial ligands.
Mice without a VDR have been shown in separate studies to be born with alopecia, an inflammatory condition in which organisms have no hair20) and age prematurely.21) Scientists have also found that Salmonella is much more virulent and aggressive in mice in which the vitamin D receptor had been turned off.22) These mice showed higher levels of activity of inflammatory molecules, and they lost weight more quickly and were much more likely to die in response to infection.
Further validating the Marshall Pathogenesis model is this: other research in VDR knockout mice has shown a marked increase, by a factor of ten, in serum 1,25-D and a clear reduction - to almost undetectable levels - in serum 25-D. Such levels persisted at seven weeks until the mice eventually died.23)
Mechanisms by which bacteria affect levels of 25-D and 1,25-D
A chronic pathogenic microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms. affects the levels of the D metabolites observed in chronic diseases in several ways. When the immune system is challenged by pathogens, the body activates CYP27B1, causing more 25-DThe vitamin D metabolite widely (and erroneously) considered best indicator of vitamin D "deficiency." Inactivates the Vitamin D Nuclear Receptor. Produced by hydroxylation of vitamin D3 in the liver. to be converted to 1,25-DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol., which, of course, increases activity of the VDRThe Vitamin D Receptor. A nuclear receptor located throughout the body that plays a key role in the innate immune response..
However, just because the concentration of 1,25-D reaches high levels - sometimes extremely high values - does not mean that the hormone is successful in binding and activating all of the body's Vitamin D Receptors (VDR). In fact, a 1,25-D that is elevated for an extended period of time suggests that the activity of the VDR is at least partially blocked.
When bacterial ligands block the VDR, the Receptor is prevented from transcribing CYP24A1, a well-studied enzyme which breaks down excess 1,25-D.
A full understanding of all these mechanisms supports the conclusion that elevated 1,25-D and depressed 25-D are a result rather than a cause of the inflammatory disease process.
Viruses and fungi also affect the VDR
- Epstein-Barr virus (EBV) – shown to downregulate expression of the VDR (and by the VDR) by a factor of about five, inducing “eventual immortalization”24) A 2011 paper further showed that a EBV not only down-regulates expression of the VDR protein itself, but also acts to block transcription by the VDR.25)
- Aspergillus fumigatus – In cystic fibrosis patients, the fungus A. fumigatus has been shown to secrete gliotoxin, a toxin which dose-dependently downregulates VDR mRNA and protein levels. This directly results in decreased levels of the AmP LL-37, thereby “providing an opportunistic environment for both bacterial and fungal colonization.”28) 29)
- Cytomegalovirus – Cytomegalovirus affects hundreds of genes including downregulating the VDR 2.2 fold.30)
- Hepatitis C virus – Gal-Tanamy et al. showed that HCV infections interfere with the VDR by inhibiting the creation of CYP24A1, the enzyme responsible for breaking down excess 1,25-D.31)
Evidence for high 1,25-D in patients with chronic disease
Under most normal conditions, serum levels of 1,25-dihydroxyvitamin DPrimary biologically active vitamin D hormone. Activates the vitamin D nuclear receptor. Produced by hydroxylation of 25-D. Also known as 1,25-dihydroxycholecalciferol, 1,25-hydroxyvitamin D and calcitirol. are constant throughout the year (no variability due to sun exposure), but there is no such tight biochemical regulation in at least some chronic inflammatory diseases such as obesity.32)
At the site of infection
It is sometimes thought that the liver and kidney are the only sites for conversion of 25-D into 1,25-D, but there is evidence that this process happens outside those organs – not coincidentally, at the very sites where patients report symptoms of chronic disease. High levels of 1,25-D and the enzyme which leads to the production of 1,25-D, 1 α-hydroxylase, have been found at various locations where the human body needs a strong host defense.33)
- skin cells of sarcoidosis patients – Sarcoidosis patients have a variety of skin symptoms including bumps, ulcers, or discolored skin. Zehnder et al found increased expression of the enzyme 1 α-hydroxylase – the enzyme which converts 25-D into 1,25-D – in the skin cells of sarcoidosis patients.34) They write:
In particular, the expression of 1α-OHase [1 α-hydroxylase] by activated macrophages and epidermal keratinocytes [skin cells] suggests a role for 1,25(OH)2D3 [1,25-D] as an immunomodulatory and/or antiproliferative hormone.
- synovial fluid surrounding the joints of patients with rheumatoid arthritis – Mawer et al found that 1,25-D levels were particularly elevated in the synovial fluid surrounding the joints of patients with rheumatoid arthritis (RA).35) In this study, median serum levels of 1,25-D at baseline was not elevated in the RA patients — only 24 pg/ml. Thus, the extrarenal synthesis of 1,25-D was not obvious from the routine blood test for 1,25-D. There is no reason to think that the metabolism of other diseases is any different.
- immune cells including macrophages – Research has also shown that 1,25-D is synthesized in cells of the immune system, including the T cells and antigen-presenting cells36) as well as the macrophages.37) 38) The fact that the immune cells are a site for 1,25-D synthesis is notable, because it is these cell types, especially macrophages, which are often infected by the Th1 pathogensThe community of bacterial pathogens which cause chronic inflammatory disease - one which almost certainly includes multiple species and bacterial forms..
There is strong evidence that the extrarenal enzyme located in macrophages plays a major role in certain granulomatous conditions (e.g., sarcoidosis), causing uncontrolled elevations of blood 1,25-(OH)2D3 levels….
Glenville et al.39)
- respiratory epithelial cells – The primary lung epithelial cells, which play an important role in the defense against airborne pathogens, have been shown to express high baseline levels of activating 1 α-hydroxylase and low levels of inactivating 24-hydroxylase. This activity leads to increased expression of genes by the Vitamin D Receptor.40)
These disease site-specific peaks in 1,25-D somewhat undercuts the validity of the 1,25-D serum blood test as the gold standard for measuring the presence of chronic disease. There is currently no clinically available whole body test for elevated 1,25-D. For this reason, the ultimate measure of diseases treatable by the Marshall ProtocolA curative medical treatment for chronic inflammatory disease. Based on the Marshall Pathogenesis. is the therapeutic probeA brief trial of the Marshall Protocol to see if it will generate an immunopathological response. The "gold standard" for testing whether a patient is a good candidate for the MP..
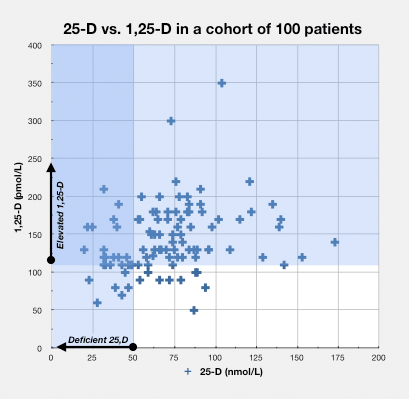
In the blood serum
As 1,25-D increases, it sometimes leaks into the bloodstream where it can be detected by measures of the metabolite in blood serum, but certainly not always.
A number of studies have demonstrated that the level of the hormone 1,25-D rises in patients with certain chronic diseases.
- autoimmune disease – Greg Blaney MD, a physician who practices in Vancouver, British Columbia (a setting with relatively infrequent sunlight), found that of a group of 100 patients with a variety of chronic inflammatory diseases, 85 had elevated measures of 1,25-D, which was defined as greater than or equal to 110 pmol/L.41)
- Crohn's disease – One study found that in a cohort of 88 Crohn's disease patients, 35 patients or 40% had elevated levels of 1,25-D, which the authors defined as above 60 pg/mL.42)
- obesity – Moan et al. showed that, in contrast to healthy people, patients with a high body mass index (BMI) had a significant seasonal variation in, not only 25(OH) vitamin D, but also of 1,25-D.43)
- sarcoidosis – Kavathia et al found that in patients with sarcoidosis, those with high serum levels of 1,25-D have more pronounced chronic treatment needs.44)
- asthma – Liu et al. showed that levels of vitamin D metabolites, particularly 1,25-D, were low within the airways and increased after allergen challenge. The increases correlated with the magnitude of inflammation and increases in cathelicidin.45)
Bell listed the following diseases and conditions, which manifest with high levels of 1,25-D: tuberculosis, AIDS with Pneumocystis carinii pneumonia, AIDS with cytomegalovirus infection, disseminated candidiasis, leprosy, rheumatoid arthritis, silicone-induced granulomas, Wegerner’s granulomatosis, Hodgkin’s disease, lymphoma, histocytic lymphoma, T-cell leukemia, plasma cell granuloma, leiomyoblastoma, seminoma, and subcutaneous fat necrosis.46)
Effect of high 1,25-D on nuclear receptors
In our study, the response to 1,25(OH)2D3 appears to be biphasic with a stimulatory effect at lower concentrations, and becoming inhibitory or ineffective at the higher levels.
Saveria Aquila et al.47)
At normal levels, the active vitamin D metabolite, 1,25-D, serves an important role in host defense,48) but high levels of the hormone are immunosuppressive49) – if for no other reason than the fact that it is calcitriol (1,25-D) and its analogues are used widely to treat autoimmune disease. One of the mechanisms by which 1,25-D may be immunosuppressive (and contribute to symptoms of disease) is by interacting with the body's other nuclear receptors. Selvaraj et al. have suggested that the high levels of 1,25-D seen in patients with pulmonary tuberculosis “might lead to downregulation of VDR expression” and that “decreased VDR levels could result in defective VDR signaling.”50) More recently, Johan Lundqvist's 2011 study showed that, consistent with the 2009 study by Proal et al. that “1α,25-dihydroxyvitamin D3 exerts tissue-specific effects on estrogen and androgen production and metabolism.”51)
Molecular modeling data show that at high levels, 1,25-D not only binds the VDR but also has a strong affinity for other key receptors that control the body's major hormonal systems including those that regulate the body's sex, thyroid, and adrenal hormones. As 1,25-D rises, it pushes out the molecules that are meant to control these receptors. Compromising these receptors can disrupt the body's ability to regulate temperature, libido, and any number of other functions.[table of affinities needed] Indeed, in the human brain, the VDR tends to be most common in the hypothalamus, which is responsible for these functions.52)
Molecular research also shows that, like the VDR, several of these nuclear receptors (including the alpha/beta thyroid receptors, glucocorticoid receptor, and androgen receptor) also express many families of antimicrobial peptides. A recent analysis of AmP expression by Brahmachary showed that the glucocorticoid receptor, the androgen receptor, and the Vitamin D Receptor, seem to be in control of 20, 17 and 16 families respectively, out of 22 analyzed.53) This means that when elevated 1,25-D displaces their endogenous ligands, the body's overall AmP expression is thwarted to an even greater degree. This further impairs the innate immune system's ability to combat chronic pathogens.
Case in point: thyroid receptor
Related article: Presentation - Vitamin D induced dysregulation of nuclear receptors may account for higher prevalence of some autoimmune diseases in women
1,25-D has a very high affinity for the alpha thyroid nuclear receptorIntracellular receptor proteins that bind to hydrophobic signal molecules (such as steroid and thyroid hormones) or intracellular metabolites and are thus activated to bind to specific DNA sequences which affects transcription. (ThRa) having a Kd value of 8.41. Normally levels of the endogenous ligand for ThRa known as T3 (which has a Kd 7.20 for ThRa) keep 1,25-D out of the binding pocket, but as 1,25-D rises due to VDR dysregulation it starts to proportionately displace T3 and block transcription by ThRa. The same thing should happen with thyroid beta – 1,25-D has a Kd of 8.44 for that receptor.
When 1,25-D displaces T3, the genes with ThRa promoters are no longer transcribed, resulting in the phenomenon known as thyroid hormone resistance. Since related nuclear receptors work as a group, when transcription by ThRa is dysregulated, system wide gene transcription is also affected.
Case in point: androgen receptor
1,25-D has a kD of 8.05 for the androgen receptor, and a Kd of 8.12 for the glucocorticoid receptor. Elevated 1,25-D can displace cortisol and testosterone from their target receptors as well, leading to an array of other hormonal imbalances.
Effect of high 1,25-D on TACO
A primary action of 1,25-D is that a high level in susceptible individuals (e.g. during pregnancy and sun holidays) causes the cellular membrane protein TACO to allow tiny bacteria to freely enter and exit the immune cells, without causing the cells to die in the process.
Dr. Andy Wright has taken photos of [bacteria freely entering and exiting cells], and it obviously would allow the pathogen(s) to spread without restraint.
Trevor Marshall, PhD
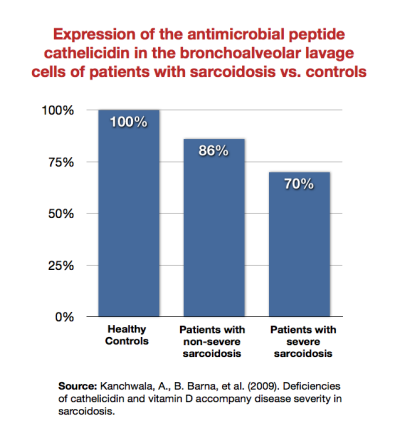
End-stage disease
As patients become sicker, their immune system becomes increasingly unable to mount a defense against infection. For example, Kanchwala et al. showed that patients with sarcoidosis expressed the antimicrobial peptide cathelicidin less than healthy subjects, and that sicker sarcoidosis patients expressed it least of all.54)
One of the hallmarks of late-stage inflammatory diseases is a very low 1,25-D with HIV/AIDS being the most commonly cited example.
HIV/AIDS
Related article: HIV and AIDS
HIV is a viral infection, and AIDS is the syndrome, which results – according to the Marshall Pathogenesis – in a dysregulated vitamin D metabolism. As evidenced by the subset of people who survive for decades with HIV, the virus itself is not necessarily deadly. Instead, it is the co-infections which are the proximate cause of the disease. One can define the breadth of AIDS-related complications by the extent and number of co-infections such as pneumonia, herpes, Candida, etc.
Supporting this hypothesis, a number of terminal AIDS patients have neglible levels of 1,25-D. 18 of 29 patients in a study of AIDS patients had undetectable levels of the metabolite.55) The patients with depressed levels of 1,25-D were characterized by advanced clinical HIV infection, low CD4+ lymphocyte counts, and high serum levels of tumor TNF-alphaA cytokine critical for effective immune surveillance and is required for proper proliferation and function of immune cells. – all indication of more severe forms of the disease.
The exact mechanism by which 1,25-D is downregulated is not entirely known, but it is highly likely that it is caused by pathogens.
Haug et al theorized that TNF-alpha and possibly other cytokinesAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. – which pathogens are known to create56) – inhibit conversion of 25-D into 1,25-D in late-stage cases of HIV/AIDS.57)
A second factor is that HIV disables D-Binding protein (DBP).58) 59) DBP is the precursor for Macrophage Activating Factor (MAF) as it has a key role to play in attracting macrophages to sites of injury. 1,25-D is transported throughout the blood by DBP.
Cancer
Related article: Vitamin D and cancer
Higher levels of CYP24A1, the enzyme which breaks down excess 1,25-D, is associated with poorer survival in lung adenocarcinoma. In a 2011 study, CYP24A1 mRNA was elevated 8-50 fold in lung cancer (compared to normal non-cancerous lung) and significantly higher in less severe cancers.60)
Lopes et al. showed that CYP24A1 expression was increased in metastatic breast cancer: 53.7% in invasive carcinomas compared to 19.0% of benign lesions.
From this study, we conclude that there is a deregulation of the Vitamin D signalling and metabolic pathways in breast cancer, favouring tumour progression. Thus, during mammary malignant transformation, tumour cells lose their ability to synthesize the active form of Vitamin D and respond to VDR-mediated Vitamin D effects, while increasing their ability to degrade this hormone.
N. Lopes,61)
Related publications and presentations
Read more research
Notes and comments
When it's ready, link to section in cancer article on hyporesponsive VDR in cancer cells.
Low levels of 25-D have been been reported in treated and untreated HIV-infected patients in Spain (86%) and in heavily pretreated patients in Italy (81.25%).(16208429, 15876279) However, a recent study from Spain found higher 25-D levels in patients receiving HAART compared to untreated patients.(16386106) Antiretroviral drug-naive patients showed lower serum levels of vitamin D metabolites and free testosterone than HAART-treated patients.
Description of Jun Sun's work: http://www.sciencedaily.com/releases/2010/07/100707141558.htm
Int J Tuberc Lung Dis. 2010 Sep;14(9):1147-1152. Serum 25-hydroxy-vitamin D(3) concentrations increase during tuberculosis treatment in Tanzania.
Tostmann A, Wielders JP, Kibiki GS, Verhoef H, Boeree MJ, van der Ven AJ.
Department of Pulmonary Diseases, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands; University Centre for Chronic Diseases, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands. Abstract
SETTING: Vitamin D deficiency is associated with susceptibility to active tuberculosis (TB) in many settings. In vitroA technique of performing a given procedure in a controlled environment outside of a living organism - usually a laboratory. studies and studies on human volunteers showed that two of the first-line anti-tuberculosis drugs, isoniazid and rifampicin, reduce 25-hydroxy vitamin D (25[OH]D) concentrations.
OBJECTIVE: To study changes in vitamin D status during treatment of Tanzanian hospitalised patients with pulmonary TB (PTB).
DESIGN: We compared serum 25[OH]D concentrations in 81 Tanzanian PTB patients before and after 2 months of treatment.
RESULTS: Median serum 25[OH]D concentrations increased from 91 nmol/l at baseline to 101 nmol/l after 2 months of TB treatment (median increase 6.0 nmol/l, IQR -0.7-25.0, P = 0.001). Median serum parathyroid hormone concentrations increased from 1.6 to 2.0 pmol/l (median increase 0.46, IQR -0.2-1.1, P < 0.001).
CONCLUSION: 25[OH]D serum concentrations increased during the first 2 months of TB treatment in 81 PTB patients in northern Tanzania. Improved dietary intake and increased sunlight exposure may have contributed to the increased 25[OH]D concentrations.
PMID: 20819260 [PubMed - as supplied by publisher]
Not sure where this should go. Here? Psoriasis? Innate immunityThe body's first line of defense against intracellular and other pathogens. According to the Marshall Pathogenesis the innate immune system becomes disabled as patients develop chronic disease.?
PLoS One. 2009 Jul 22;4(7):e6340. Vitamin D analogs differentially control antimicrobial peptide/“alarmin” expression in psoriasis.
Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Büchau A, Steinmeyer A, Zügel U, Ruzicka T, Schauber J. Department of Dermatology and Allergology, Ludwig-Maximilians-University, Munich, Germany. Abstract Antimicrobial peptides (AMPs) are strongly expressed in lesional skin in psoriasis and play an important role as proinflammatory “alarmins” in this chronic skin disease. Vitamin D analogs like calcipotriol have antipsoriatic effects and might mediate this effect by changing AMP expression. In this study, keratinocytes in lesional psoriatic plaques showed decreased expression of the AMPs beta-defensin (HBD) 2 and HBD3 after topical treatment with calcipotriol. At the same time, calcipotriol normalized the proinflammatory cytokineAny of various protein molecules secreted by cells of the immune system that serve to regulate the immune system. milieu and decreased interleukin (IL)-17A, IL-17F and IL-8 transcript abundance in lesional psoriatic skin. In contrast, cathelicidin antimicrobial peptide expression was increased by calcipotriol while psoriasin expression remained unchanged. In cultured human epidermal keratinocytes the effect of different vitamin D analogs on the expression of AMPs was further analyzed. All vitamin D analogs tested blocked IL-17A induced HBD2 expression by increasing IkappaB-alpha protein and inhibition of NF-kappaBA protein that stimulates the release of inflammatory cytokines in response to infection signaling. At the same time vitamin D analogs induced cathelicidin through activation of the vitamin D receptor and MEK/ERK signaling. These studies suggest that vitamin D analogs differentially alter AMP expression in lesional psoriatic skin and cultured keratinocytes. Balancing AMP “alarmin” expression might be a novel goal in treatment of chronic inflammatory skin diseases.
PMID: 19623255
J Microbiol Immunol Infect. 2008 Feb;41(1):17-25. Synergistic action of vitamin D and retinoic acid restricts invasion of macrophages by pathogenic mycobacteria.
Anand PK, Kaul D, Sharma M. Molecular Biology Unit, Department of Experimental Medicine and Biotechnology, Post Graduate Institute of Medical Education and Research, Chandigarh, India. Abstract BACKGROUND AND PURPOSE: Phagosomal maturation arrest is known to play a central role in the survival of pathogenic mycobacteria within macrophages. The maturation arrest of mycobacterial phagosome results from the retention of tryptophan-aspartate-containing coat protein (TACO) on this organelle, enabling successful replication of the pathogen. We have shown earlier that vitamin D(3) and retinoic acid (RA) down-regulate TACO gene transcription in a dose-dependent manner. METHODS: In this study, we analyzed the promoter region of TACO gene using bioinformatics tools and observed that the vitamin D receptor (VDR)/retinoid-X-receptor (RXR) response sequence was highly functional. We also evaluated the effect of treatment with vitamin D(3)/RA on Mycobacterium tuberculosis entry and survival in cultured human macrophages. RESULTS: TACO gene down-regulation observed with vitamin D(3)/RA treatment occurred through modulation of this gene via the VDR/RXR response sequence present in the promoter region of TACO gene. Treatment of macrophages with vitamin D(3)/RA allows maturation of mycobacterial phagosome, leading to degradation of the pathogen. CONCLUSIONS: Our results elucidate the mechanism of TACO gene down-regulation observed with vitamin D(3)/RA. Furthermore, the results revealed that vitamin D(3)/RA treatment inhibits mycobacterial entry as well as survival within macrophages, possibly through rescue of phagosome maturation arrest. The developing knowledge in this area suggests that vitamin D(3)/RA may be of importance in the treatment of intracellular infection, particularly tuberculosis. PMID: 18327422
The more we understand about gene transcription by VDR the more complex it is becoming. Again we are asymptoting towards infinite-complexity as we learn more and more about how transcription actually works… For a very long time it has been known that EBV delays cell-death, an obvious way of allowing the microbiotaThe bacterial community which causes chronic diseases - one which almost certainly includes multiple species and bacterial forms. to form stable communities within human cells. Here is a 1991 paper:
http://www.ncbi.nlm.nih.gov/pubmed/1659776
Another early paper showed Sarcoidosis was also associated with reduced cell-death (I met one of the authors of this paper last week at the Microbiome conference, and he was surprised when I told him how important it had been in helping me formulate the early MP pathogenesis):
http://www.smw.ch/docs/pdf200x/2001/31/smw-09808.pdf
Well, a new paper is out showing that EBV not only down-regulates expression of the VDR protein itself, but also acts to block transcription by the VDR:
http://www.ncbi.nlm.nih.gov/pubmed/20593215
Here are some of the key points from the fulltext of this paper: “In this study, we found that EBNA-3 and VDR are in the same protein complex. EBNA-3 blocks (or downregulates) transcription of the VDR-dependent genes at the basal level and upon its activation by a ligand, the 1-alpha,25-dihydroxyvitamin D3. Binding between EBNA-3 and VDR prevents LCLs from growth arrest and/or apoptosis induced by vitamin D3Form of vitamin D made in the skin when exposed to light. Also available in fish and meat. This secosteroid is sometimes converted into 25-D. Also known as cholecalciferol and activated 7-dehydrocholesterol.”
“The effective dose of 1,25(OH)2D3 required to initiate VDR response is in the range of 1–10 nM”
“The transactivating ability of VDR is enhanced by binding to its ligand, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3)”
“The concentration of 1 9 10-7M of 1,25(OH)2D3 that was used in our experiments was higher when compared to the physiological level of 1,25(OH)2D3. Even at such concentrations of 1,25(OH)2D3, EBNA-3 could protect LCLs from VDR-induced apoptosis.”
“In summary, we have found that EBNA-3 protein is one of the units of the nuclear VDR protein complex. Notably, we have found that EBNA-3 participates in complex formation with other proteins that can regulate transcription by chromatin re-modeling, such as SWI/SNF, DNA helicase II, and histones 2A and 2B. We can hypothesize that, when EBNA-3 is bound to VDR, it represses VDR-dependent transcription and protects LCLs from VDR-induced growth arrest and/or apoptosis.”
Those of you who really want to dig into the details will need to get a copy of the fulltext, but I think the above, plus the abstract, are the key points.
Note particularly the use of the word “enhanced” when talking about the activation of the VDR by 1,25-D. It is currently thought that VDR can express some genes without any ligand in its binding pocket. Pretty weird stuff, difficult to study in a “on/off” environment, truly needing a stochastic methodology. Traditional concepts of receptor “antagonists” and “agonists” are already starting their journey towards the junk-heap…
..Trevor..
EBV does both - down-regulate expression of and expression by the VDR.
Trevor
Couldn't find a cite for this statement: “Not a lot is known yet about the complexities of DBP/MAF, but mice which have no DBP/MAF also have a zero level of 1,25-D measurable in the bloodstream and are very susceptible to infection. Trevor”
It is known that D Binding Protein (Macrophage Activating Factor) is modified in chronic disease, and yet this protein is the sole carrier of 1,25-D in the bloodstream. Without DBP there is no measurable 1,25-D and the patient is very susceptible to an infection (from loss of the MAF function).
I know it seems like nothing is simple these days, and indeed that is getting more and more the case. But the data I am seeing indicates that 1,25-D RAI has not been measuring what we think it is measuring.
At this point I have deprecated all diagnostic specificity of 1,25-D testing until we get a better handle on what is happening. This prevents people being declared “healthy” because of an erroneously low 1,25-D value. As time goes by we will probably come to understand why the blood from chronically ill measures so differently from the blood of healthy subjects.
Meanwhile, for the 40pg/ml cutoff value to be useful, the testing must be performed by RAI on a sample which has been frozen during transit. Trevor
A section on vitamin D binding protein would be good. Some of this could be included:
Mol Immunol. 2011 Dec;49(3):495-503. Epub 2011 Oct 19. Cofactor regulation of C5a chemotactic activity in physiological fluids. Requirement for the vitamin D binding protein, thrombospondin-1 and its receptors. Trujillo G, Zhang J, Habiel DM, Ge L, Ramadass M, Ghebrehiwet B, Kew RR. Source Department of Pathology, Stony Brook University School of Medicine, Stony Brook, NY 11794-8691, USA. Abstract Factors in physiological fluids that regulate the chemotactic activity of complement activation peptides C5a and C5a des Arg are not well understood. The vitamin D binding protein (DBP) has been shown to significantly enhance chemotaxis to C5a/C5a des Arg. More recently, platelet-derived thrombospondin-1 (TSP-1) has been shown to facilitate the augmentation of C5a-induced chemotaxis by DBP. The objective of this study was to better characterize these chemotactic cofactors and investigate the role that cell surface TSP-1 receptors CD36 and CD47 may play in this process. The chemotactic activity in C-activated normal serum, citrated plasma, DBP-depleted serum or C5 depleted serum was determined for both normal human neutrophils and U937 cell line transfected with the C5a receptor (U937-C5aR). In addition, levels of C5a des Arg, DBP and TSP-1 in these fluids were measured by RIA or ELISA. Results show that there is a clear hierarchy with C5a being the essential primary signal (DBP or TSP-1 will not function in the absence of C5a), DBP the necessary cofactor and TSP-1 a dependent tertiary factor, since it cannot function to enhance chemotaxis to C5a without DBP. Measurement of the C5a-induced intracellular calcium flux confirmed the same hierarchy observed with chemotaxis. Moreover, analysis of bronchoalveolar lavage fluid (BALF) from patients with the adult respiratory distress syndrome (ARDS) demonstrated that C5a-dependent chemotactic activity is significantly decreased after anti-DBP treatment. Finally, results show that TSP-1 utilizes cell surface receptors CD36 and CD47 to augment chemotaxis, but DBP does not bind to TSP-1, CD36 or CD47. The results clearly demonstrate that C5a/C5a des Arg needs both DBP and TSP-1 for maximal chemotactic activity and suggest that the regulation of C5a chemotactic activity in physiological fluids is more complex than previously thought.
Copyright © 2011 Elsevier Ltd. All rights reserved.
PMID: 22014686
Diabetes. 2011 Oct;60(10):2566-70. Epub 2011 Aug 15. Reduced serum vitamin d-binding protein levels are associated with type 1 diabetes. Blanton D, Han Z, Bierschenk L, Linga-Reddy MV, Wang H, Clare-Salzler M, Haller M, Schatz D, Myhr C, She JX, Wasserfall C, Atkinson M. Source Department of Pathology, University of Florida, Gainesville, Florida, USA. Abstract OBJECTIVE: Previous studies have noted a specific association between type 1 diabetes and insufficient levels of vitamin D, as well as polymorphisms within genes related to vitamin D pathways. Here, we examined whether serum levels or genotypes of the vitamin D-binding protein (VDBP), a molecule key to the biologic actions of vitamin D, specifically associate with the disorder.
RESEARCH DESIGN AND METHODS: A retrospective, cross-sectional analysis of VDBP levels used samples from 472 individuals of similar age and sex distribution, including 153 control subjects, 203 patients with type 1 diabetes, and 116 first-degree relatives of type 1 diabetic patients. Single nucleotide polymorphism (SNP) typing for VDBP polymorphisms (SNP rs4588 and rs7041) was performed on this cohort to determine potential genetic correlations. In addition, SNP analysis of a second sample set of banked DNA samples from 1,502 type 1 diabetic patients and 1,880 control subjects also was used to determine genotype frequencies.
RESULTS: Serum VDBP levels were highest in healthy control subjects (median 423.5 µg/mL [range 193.5-4,345.0; interquartile range 354.1-]586), intermediate in first-degree relatives (402.9 µg/mL [204.7-4,850.0; 329.6-492.4]), and lowest in type 1 diabetic patients (385.3 µg/mL [99.3-1,305.0; 328.3-473.0]; P = 0.003 vs. control subjects). VDBP levels did not associate with serum vitamin D levels, age, or disease duration. However, VDBP levels were, overall, lower in male subjects (374.7 µg/mL [188.9-1,602.0; 326.9-449.9]) than female subjects (433.4 µg/mL [99.3-4,850.0; 359.4-567.8]; P < 0.0001). It is noteworthy that no differences in genotype frequencies of the VDBP polymorphisms were associated with serum VDBP levels or between type 1 diabetic patients and control subjects.
CONCLUSIONS: Serum VDBP levels are decreased in those with type 1 diabetes. These studies suggest that multiple components in the metabolic pathway of vitamin D may be altered in type 1 diabetes and, collectively, have the potential to influence disease pathogenesis.
PMID: 21844098
J Neurol. 2011 Mar;258(3):353-8. Epub 2010 Nov 2. The emerging role of vitamin D binding protein in multiple sclerosis. Disanto G, Ramagopalan SV, Para AE, Handunnetthi L. Source Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Headington, Oxford OX3 7BN, UK. Abstract Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system (CNS). A growing body of evidence supports a role for vitamin D in MS aetiology. Vitamin D binding protein (DBP) is the major plasma carrier of vitamin D metabolites and genetic differences in DBP gene have been found to influence vitamin D levels. We review here evidence supporting a role of DBP in MS. Several recent studies show that DBP levels in the cerebrospinal fluid correlate with MS course, being lower during relapses and higher in the secondary progressive phase. Further studies are needed to elucidate the potential use of DBP as a biological marker of MS course, but may be of use given the current lack of diagnostic tools for the prediction of MS development and progression.
PMID: 21042807
Front Biosci (Elite Ed). 2010 Jun 1;2:796-804. Proteomics reveals high levels of vitamin D binding protein in myocardial infarction. Gasparri C, Curcio A, Torella D, Gaspari M, Celi V, Salituri F, Boncompagni D, Torella M, Gulletta E, Cuda G, Indolfi C. Source Division of Cardiology, Magna Graecia University, Catanzaro, Italy. Abstract The pathogenic mechanisms underlying cardiovascular diseases involve significant alterations in myocardial gene and protein expression. Proteomics analysis can define new protein and peptide changes associated with cardiac pathology, including myocardial infarction. The aim of the present study was to analyze serum proteome of patients with ST-Elevation myocardial infarction (STEMI). Serum samples were collected from STEMI patients (age 65.0+/-10.3) at 5.3+/-2.7 hours after the onset of typical chest pain and before initiating standard therapy. Ten age- and sex-matched donors were used as controls. The samples were albumin- and IgG-depleted. Isotope-coded affinity tag method was employed to label cysteine residues and liquid chromatography-Tandem Mass Spectrometry analysis was performed to measure the labeled proteins. Our proteomic approach identified increased levels of vitamin D-binding protein precursor (VDB) in the serum of STEMI patients when compared to control donors. Western blot analysis confirmed the increase in VDB protein in STEMI patients. Moreover, fresh thrombotic plaques, obtained during primary angioplasty, showed high expression of VDB protein. Mechanistically, VDB protein reduces platelet aggregation and prolongs coagulation time ex vivo.
PMID: 20515752
Mol Immunol. 2007 Mar;44(9):2370-7. Epub 2006 Nov 20. Upregulation of vitamin D binding protein (Gc-globulin) binding sites during neutrophil activation from a latent reservoir in azurophil granules. DiMartino SJ, Trujillo G, McVoy LA, Zhang J, Kew RR. Source Department of Pathology, Stony Brook University School of Medicine, Stony Brook, NY 11794-8691, USA. Abstract Vitamin D binding protein (DBP) is a multifunctional plasma transport protein that is also found on the surface of many cell types. Cell surface DBP significantly enhances chemotactic activity of complement (C) peptides C5a and C5a des Arg. However, both DBP binding and C5a chemotaxis enhancement can vary among neutrophil donors. To test if activation during cell purification is responsible for this variability, neutrophils were isolated using both standard and lipopolysaccharide (LPS)-free protocols. Cells isolated by the LPS-free method had no DBP-enhanced chemotaxis to C5a or DBP binding to plasma membranes. Moreover, neutrophils treated with LPS bound more avidity to immobilized DBP than sham-treated cells. Subcellular fractionation of neutrophils (standard protocol) revealed a heavy plasma membrane (HM) band that contained components of light plasma membranes and all three granules. The HM band possessed most of the DBP binding activity (58%), and activation of cells with ionomycin greatly increased DBP binding to HM. Azurophil granules contained 33% of the total DBP binding sites and there was a highly significant positive correlation (r=0.988) between release of the granule marker myeloperoxidase and DBP binding. These results indicate that fusion of granules with the plasma membrane forms HM that contains DBP binding sites.
PMID: 17113648
Vitamin D Binding Protein-Macrophage Activating Factor Directly Inhibits Proliferation, Migration, and uPAR Expression of Prostate Cancer Cells. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2956649/?tool=pubmed